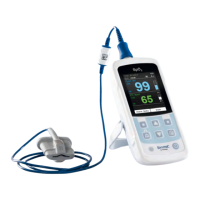
Pulse oximeter MySign
®
S
3 Designated use and functional description
3.1 Indications for use (designated purpose)
MySign
®
S is a handheld pulse oximeter with accessory sensors indicated for continuous non-invasive
monitoring of the functional oxygen saturation of arterial hemoglobin (SpO2) and pulse rate for adult and
pediatric (excluding neonatal and infant) patients in hospitals, hospital-type facilities, and mobile units.
For professional use only.
The areas in the hospital where the unit can be used include general nursing wards, operating rooms,
areas for special procedures and intensive care units both in hospitals and in hospital-like institutions.
Hospital-like areas include, for example, out-patient clinics, sleep laboratories, care facilities, surgical
centers and day clinics.
MySign
®
S allows for comprehensive basic monitoring of SpO2 and pulse rate including:
• Configuring of upper and lower alarm limits
• Standard alarm limits
• Visual and audible monitoring
The monitor provides retrospective access to the monitoring data via a USB connection to a PC
utilizing the optional MySign PC Software.
The data is stored by the monitor in a trend and event database. It is possible to display trend tables
(vital parameters), store them on a central server for documentation or print them out at any location.
The measurements can also be displayed as a trend diagram.
The sensors are applied to the corresponding body parts of the patient, such as the finger. Based on
the measurement values, pulse-oximetric oxygen saturation (SpO2), pulse rate, pulsation index and
 Loading...
Loading...