Pulse oximeter MySign
®
S
13.1 Clinical Accuracy
The MySign
®
S has been clinically evaluated for accuracy in accordance with ISO 80601-2-61. Testing was
performed in healthy adult volunteers providing informed consent in an institutionally approved clinical protocol,
with arterial blood samples measured by co-oximetry as the reference samples.
The MySign
®
S has been clinically evaluated with the following SpO2 sensor types:
• F-3212-9 Reusable FingerClip SpO2 Sensor
• ES-3212-9 Reusable EarClip SpO2 Sensor
• R-3212-9 SoftTip Reusable Rubber Finger Sensor
• DW-2211-6 Disposable Adhesive Tape Sensor
• DS100A Nellcor Finger Clip Sensor (NOTE: third party sensor not manufactured by EnviteC)
In total the sensor types above are representative of the construction of the MySign
®
S full sensor product offerings
for FingerClip, EarClip, SoftTip, and disposable sensors. The reported accuracy for each sensor type is listed in
the table and in the graphical plots below.
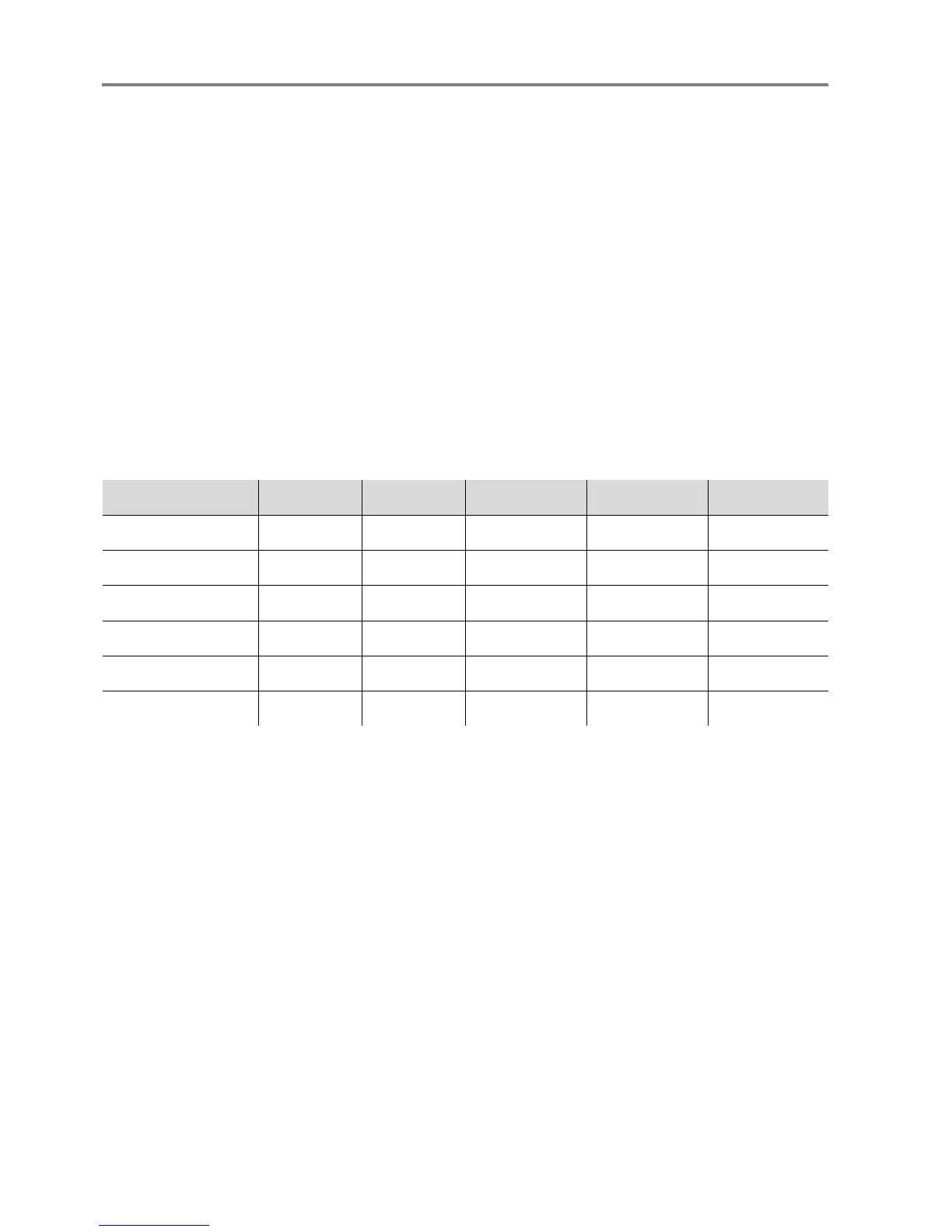
Parameter F-3212-9 ES-3212-9 R-3212-9 DW-2211-6 DS100A
A
RMS
70-80% 2.35 2.90 1.85 2.41 2.41
A
RMS
80-90% 2.09 1.33 1.73 2.14 1.44
A
RMS
90-100% 1.50 1.33 1.40 1.90 1.48
A
RMS
1
70-100% 1.86 1.98 1.60 2.01 1.79
Upper 95% LOA
2
1.673 3.937 2.063 2.331 3.989
Lower 95% LOA -4.050 -3.888 -3.559 -4.465 -2.444
1. A
RMS
is root mean square error. This value is expected to contain about 2/3 of the oximeter.
2. LOA is Limits of Agreement – expresses the 95% confidence interval upper and lower boundaries.
Graphical error plots for each sensor type are provided below, and have been constructed with reference to BLAND,
J. M., ALTMAN, D. G. Agreement Between Methods of Measurement with Multiple Observations Per Individual.
Journal of Biopharmaceutical Statistics, 17:4, 571 – 582
Refer to the respective user manuals for each sensor type for more information.
46 066-07-1002197_GA_MySignS_FDA / 06.14
 Loading...
Loading...