ALYA INSTALLATION AND USER’S MANUAL - EN
Edition 2.0 Sept 2018 Pag. 24 di 32
7 STERILIZATION OF THE HANDLES
Warning - danger of cross contamination
The handles are not supplied sterile, they must therefore be sterilised before use. The
handles must be sterilised before each patient.
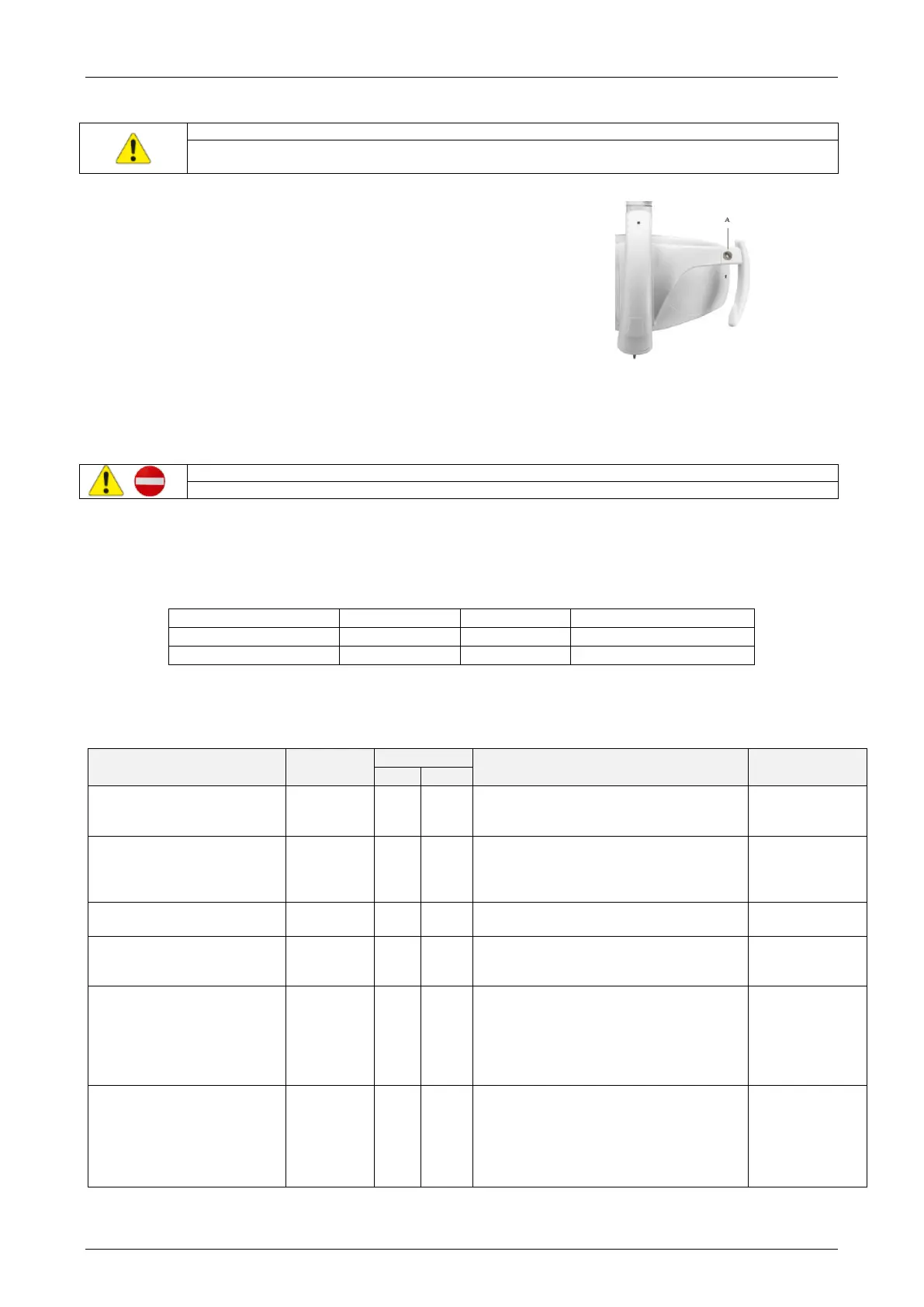
7.1 Removal of the handle
To remove the handle, unscrew knob “A” and remove it from the
support.
7.2 Decontamination and disinfection
Before sterilising the handles, they must be decontaminated and disinfected.
To disinfect, Faro has tested the following products for disinfection: Eco Jet-1 (Cattani Group) / Sporekin
Plus DS (Ims srl) / Zefirol Quick (Molteni Dental) / Durr FD366 Sensitive
Attention - danger of plastic breaking
The handles cannot be disinfected by thermo-disinfection.
7.3 Sterilization
The handles must be packaged in compliance with EN 868-5.
The handles can be sterilised with standard cycles 121°/134° C up to two hundred (200) cycles or however
up to loss of the mechanical performance.
The parameters of the sterilisation cycle are as follows:
Minimum cycles of sterilisation for mechanical integrity: 200
8 PERIODIC CHECKS
Check the absence
of any play between the
arm joints
Verify the play between the joints
Check the absence
of any oxidation into
joints, arms or plastic
parts.
Check the main plate can
be read
Check of damages on
enclosure and plastic
joints integrity
Electrical Safety according
EN 62353
1. Dielectric strenght
2. Current Leakage.
Use the parameters defined into IEC
60601-1
With a spectroradiometer check the
values for:
Max Luminance: >35000 lux
CRI decay: <20%.
Radial power on blue light: <100
W/m2
Service Engineer: competent person qualified to mantain, check and repair powered Medical Devices and Systems.

 Loading...
Loading...