System Glucose Availability
The System is designed to generate a GM value every 15 minutes throughout the sensor wear time. Overall, 202
sensors were inserted. 167 sensors produced glucose readings and are included in the analysis. There were
35 sensors that failed at insertion (i.e. no glucose reading generated) and are not included in the analysis. There
were 62.5% of primary sensors that worked for 14 days. The mean sensor duration for all primary sensors was
determined to be 258 hours, and the median duration of was 327 hours.
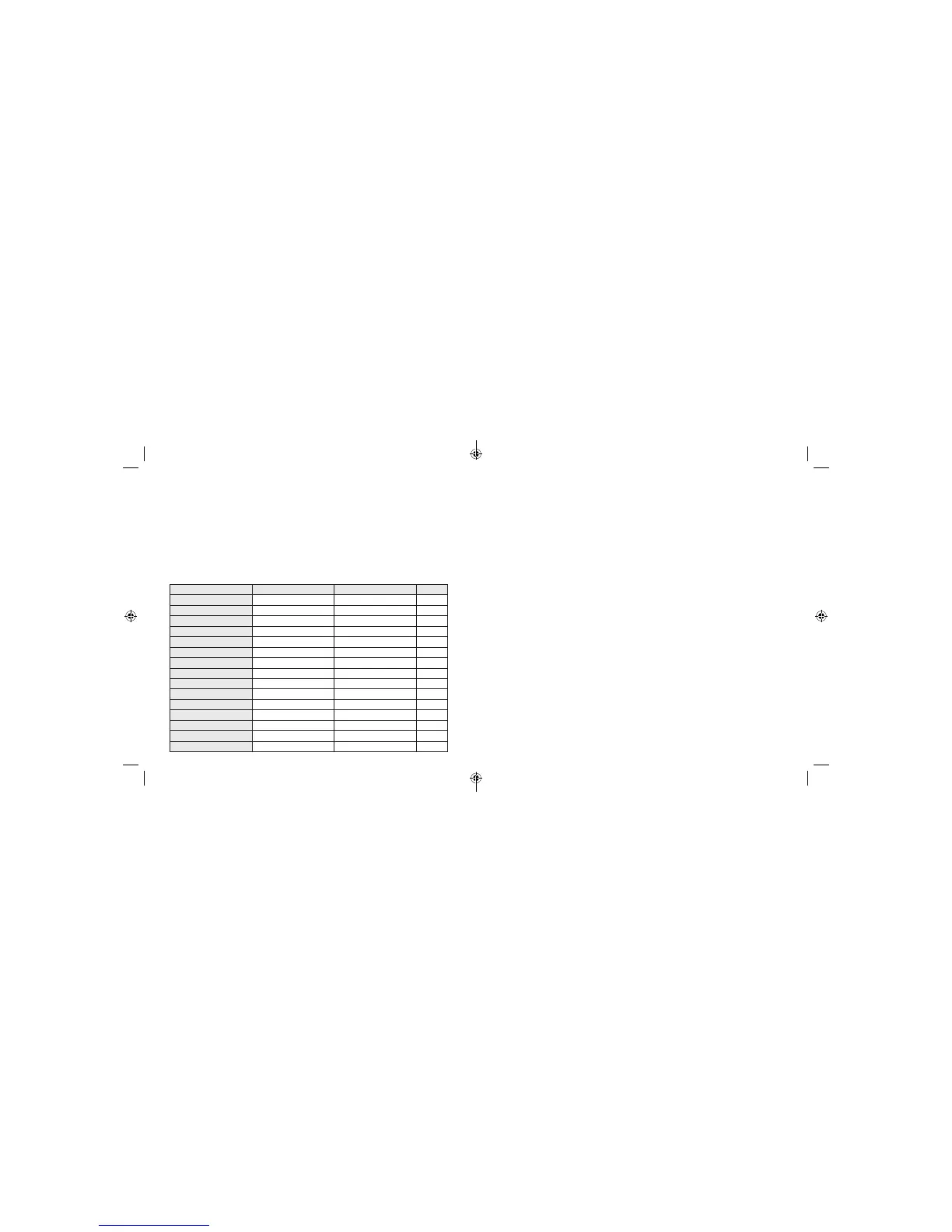
Table 14 shows the number of available glucose readings reported by all sensors (by sensor operational hour)
that produced at least one GM reading during the clinical study over the 14-day wear period. The percentage of
available GM readings is presented in comparison to the number of expected GM readings based on the number of
hours of sensor wear. Overall, 96.9% (153,169 GM readings out of expected 158,052) of GM readings were available.
Table 14: GM Availability
Operational Hour No. Historic GM Expected No. %
0 - 24 81 83 97.6
24 - 48 795 813 97.8
48 - 72 1170 1201 97.4
72 - 96 1053 1080 97.5
96 - 120 1187 1230 96.5
120 - 144 2630 2725 96.5
144 - 168
3262
3356 97.2
168 - 192
4478
4617 97.0
192 - 216
5101
5223 97.7
216 - 240
3413
3531 96.7
240 - 264
5611
5759 97.4
264 - 288
7021
7184 97.7
288 - 312
6300
6400 98.4
312 - 336
111067
114850 96.7
Overall
153169
158052 96.9
Precision
Precision of the System was evaluated by comparing the results from two separate sensors worn on
the same subject at the same time. Data from two sensors worn at the same time for 72 subjects
provided 49,806 pairs of GM measurements. The mean PARD during the study was 8.6% with a
coecient of variation of 6.1%.
Sensor Wear Duration
Sensors may be worn for up to 14 days (≥324 hours). To estimate how long a sensor will work over
14 days, 34 sensors were evaluated to determine how many days of readings each sensor provided.
Results show that 85.3% of sensors lasted for the intended 14-day wear duration.
Adverse Events
No device-related serious adverse events occurred during the study. Mild skin irritation, such as
erythema, edema, rash, bleeding, itching, bruising, scaling skin, and induration were reported
around the insertion site and adhesive area by a moderate frequency of subjects (26 out of 72 or
36%). Pain was mostly reported as none with only one reported instance of mild pain.
62 63
ART26944_rev-C_manual.indd 63 9/23/16 9:19 AM
 Loading...
Loading...