1
Congratulations!
Thank you for buying a Milli-Q
®
water purication system.
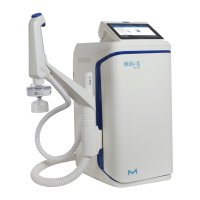
Milli-Q
®
EQ 7000 produces ultrapure water from a puried water source. Installation of this
product should be performed by a qualied service representative with access to qualied
installation documentation.
This user manual is a guide for use during the normal operation and maintenance of a
Milli-Q
®
EQ 7000 water purication system. It is highly recommended to fully read this manual
and understand its content before handling the water purication system.
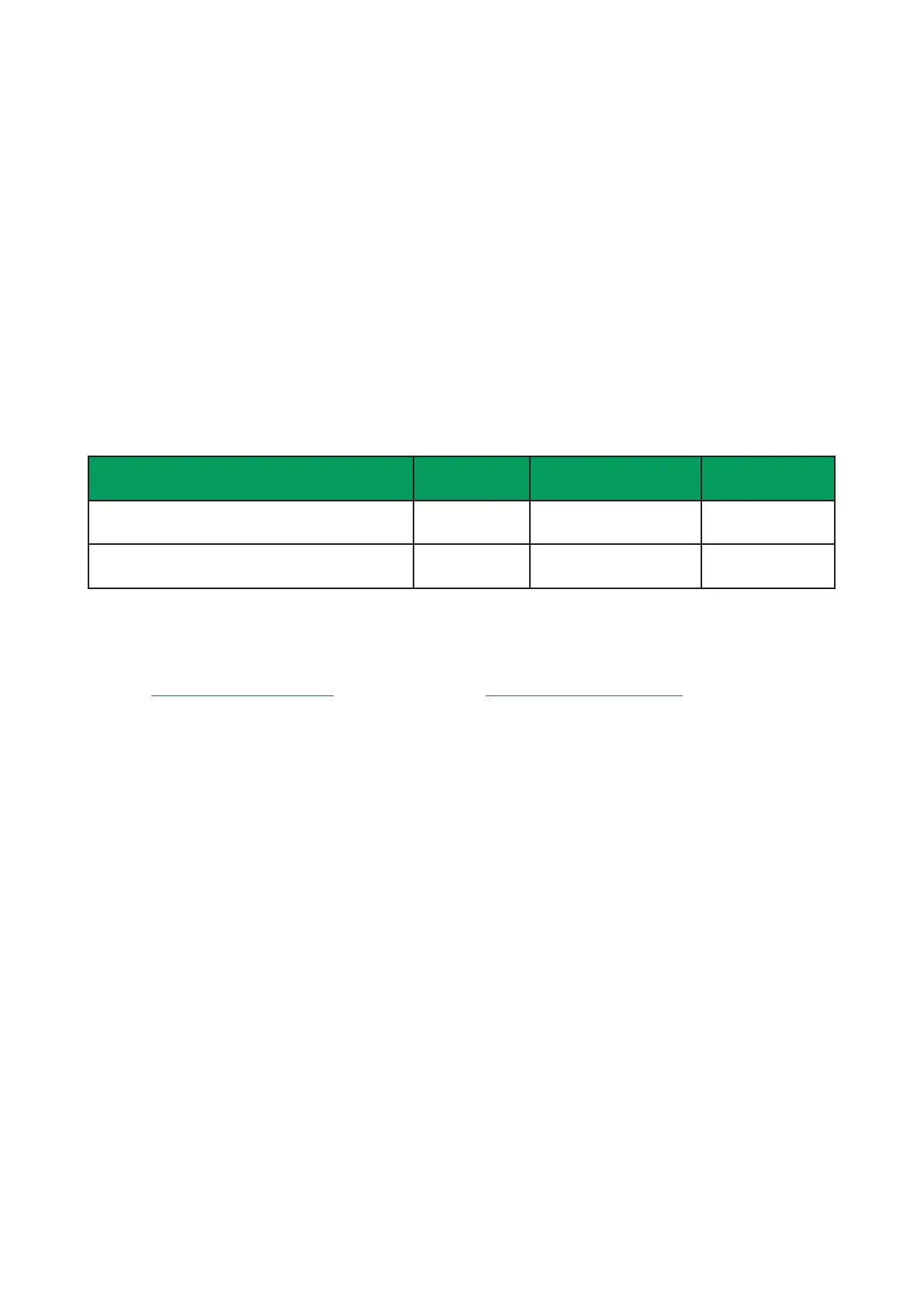
System identication
System
Catalog
number
Voltage Frequency
Milli-Q
®
EQ 7000 with local dispenser ZEQ7000T0 100-240 Vac ±10% 50-60 Hz ±2Hz
Milli-Q
®
EQ 7000 with remote dispenser ZEQ7000TR 100-240 Vac ±10% 50-60 Hz ±2Hz
Manufacturing site:
Millipore SAS, 67120 Molsheim, France
For more information on your Milli-Q system, please call your local representative or visit our
website www.emdmillipore.com (North America) or www.merckmillipore.com (Rest of the World).
Intended use
The Milli-Q
®
EQ 7000 is intended to produce ultrapure water from a puried water source
primarily for use in a variety of laboratories worldwide.
The product is designed to produce ultrapure water with specic characteristics (refer
to the requirements and specications section), provided that it is fed with water
quality within specications and it is properly maintained as required by the supplier.
Merck KGaA does not warrant the product for any specic application. It is up to the user
to determine if the quality of the water produced matches their expectations, ts with
norms/legal requirements and to bear responsibility resulting from the usage of the water.
The product is not intended to produce: water for injection, water for dialysis, sterile water for
irrigation or injection, bacteriostatic water for injection, sterile puried water in containers, and
sterile water for injection in container or ingestion. The product is not intended to be used in
explosive environments according to ATEX Directive – equipment & protective systems intended
for use in potentially explosive atmospheres. In addition the product is not intended as a Medical
Device, including In-Vitro Devices.
INTRODUCTION
 Loading...
Loading...