Introduction Notes
viii 0070-10-0603-01 Duo™ Operating Instructions
Notes
NOTE: Potential hazards due to errors in software or hardware
have been minimized by actions taken in accordance with
IEC 60601-1-4.
NOTE: Information codes and error codes with corresponding
explanations are provided to assist in the identification and
correction of problems that may occur with the monitor.
NOTE: The comparison testing conducted via the auscultatory
method used both Phase 4 and Phase 5 Korotkoff sounds. A
report of the study finding for the auscultatory method is
available by contacting Technical Support (201) 995-8116.
NOTE: The use of this equipment is restricted to one patient at a
time.
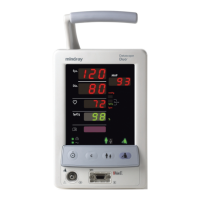
Indication for Use
The Duo monitor is intended for use in health care settings under the direct supervision of a
licensed health care practitioner. The intended use of the monitor is to monitor physiologic
parameter data on adult and pediatric patients. Physiologic data includes: non-invasive
blood pressure (NIBP), pulse oximetry and pulse rate as summarized in the operating
instructions manual. The information can be displayed only. The monitor is not intended for
home use.
Unpacking
Remove the instrument and accessories from the shipping cartons and examine them for signs
of damage. Check all materials against the packing list. Save the invoice, bill of lading and
all packing materials. These may be required to process a claim with the carrier. Contact a
Sales Representative or Distributor for assistance in resolving shipping problems.
 Loading...
Loading...