16 - 38 Operator’s Manual
16 Probes and Biopsy
3. Prepare a sterilant by using sterile distilled water when necessary.
4. Immerse the needle-guided bracket fully in the sterilant and shake the bracket appropriately to
remove any bubbles on the surface. Use a syringe to draw an appropriate amount of sterilant
and inject the sterilant into the hole to remove the bubbles inside the hole if necessary.
For details about the immersion duration, see the operator’s manual provided by the
manufacturer.
5. After sterilization, wash the needle-guided bracket thoroughly by using a large amount of
sterile distilled water (about 2 gallons) at room temperature for about 30 s to remove the
residual sterilant. Repeat the operation twice.
6. Dry the needle-guided bracket by using a piece of sterile disposable lint-free soft cloth.
7. Store the needle-guided bracket in a cool, clean and dry environment.
High-pressure steam sterilization
High-pressure steam sterilization is preferred for the metal guided brackets. And the sterilizer and
accessories should be cleared by FDA for the intended sterilization cycle.
Perform the following procedure:
1. Wear a pair of gloves to prevent infection.
2. Clean thoroughly in accordance with the cleaning procedure before sterilization.
3. Package the needle-guided bracket in accordance with the sterilization requirements of
surgical instruments using the sterilization wrap or pouch cleared by FDA.
4. Place the packaged needle-guided bracket inside a high-temperature steam sterilizer and
perform sterilization. The sterilization parameters are 121 °C and 30 minutes for a gravity
displacement steam sterilizer and are 132 °C and 4 minutes for a dynamic-air-removal steam
sterilizer.
5. Take out the sterilization package after sterilization and dry it in an oven at 60 °C for 20
minutes to 30 minutes.
Keep the sterilization package together with other sterilized surgical instruments in a sterile
item storage area.
16.2.6 Storage and Transportation
• Do not use the carrying case for storing the needle-guided bracket. If the carrying case is used
for storage, it may become a source of infection.
• Between examinations, keep the needle-guided bracket in a sterile environment.
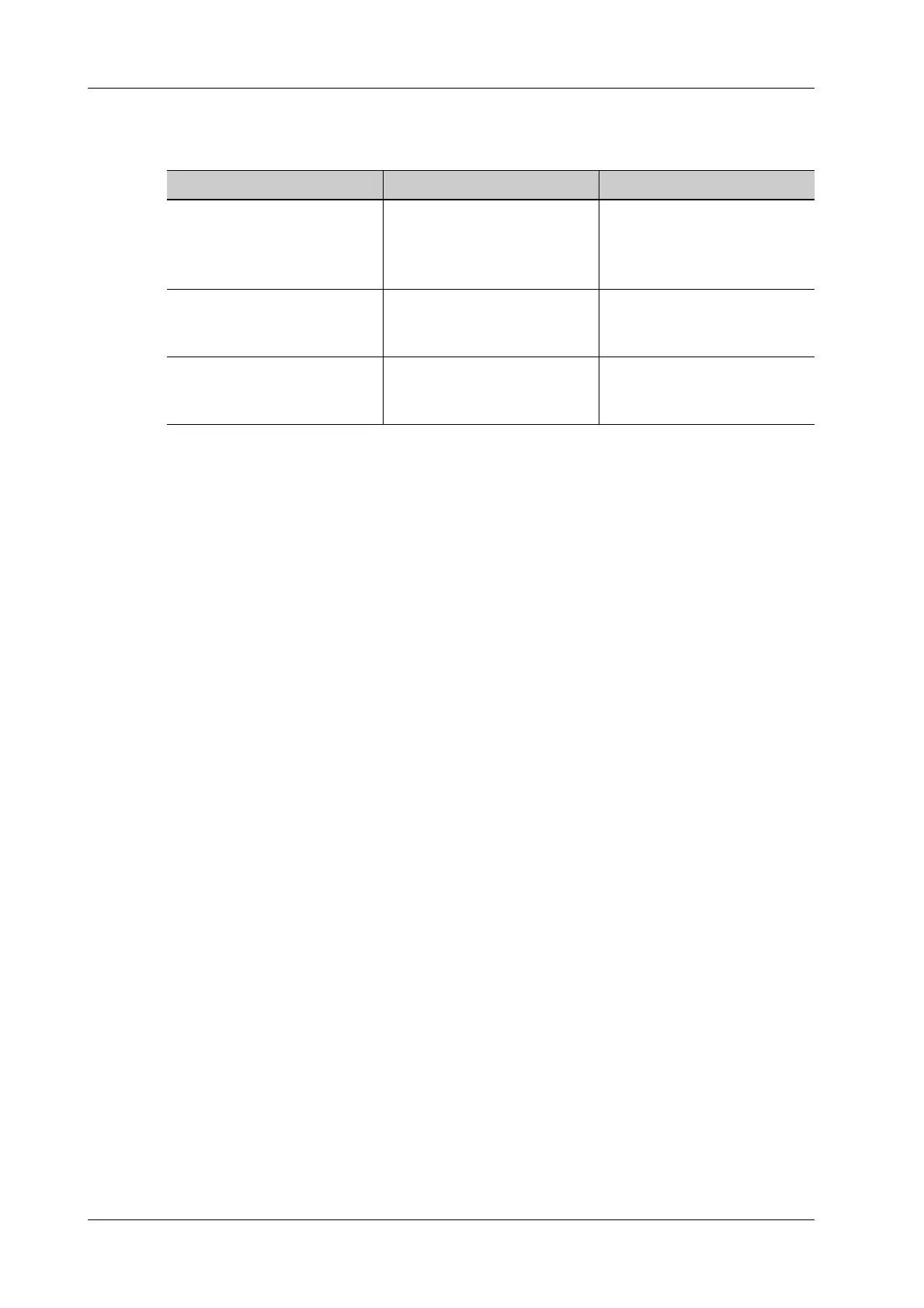
Table 16-5 Recommended sterilization solution
Chemical name Trade name Procedures
Glutaraldehyde (2.4%) Cidex Activated Dialdehyde
Solution
(applicable for FDA region
only)
Refer to the instructions
provided by the solution
manufacturer for details.
22% Hydrogen Peroxide
4.5% Peroxyacetic Acid
Minncare liquid disinfectant
(applicable for Canada only)
Refer to the instructions
provided by the solution
manufacturer for details.
Glutaraldehyde (2.6%) Metricide Refer to the instructions
provided by the solution
manufacturer for details.
 Loading...
Loading...