4: Sample Experiments
Absorbance Experiments
Absorbance spectra are a measure of how much light is absorbed by a sample. For most samples,
absorbance is linearly related to the concentration of the substance. The software calculates absorbance
(Al) using the following equation:
S
λ
- D
λ
A
λ
= -
log
10
(
R
λ
-
D
λ
)
Where:
S
λ
= the sample intensity at wavelength λ
D
λ
= the dark intensity at wavelength λ
R
λ
= the reference intensity at wavelength λ
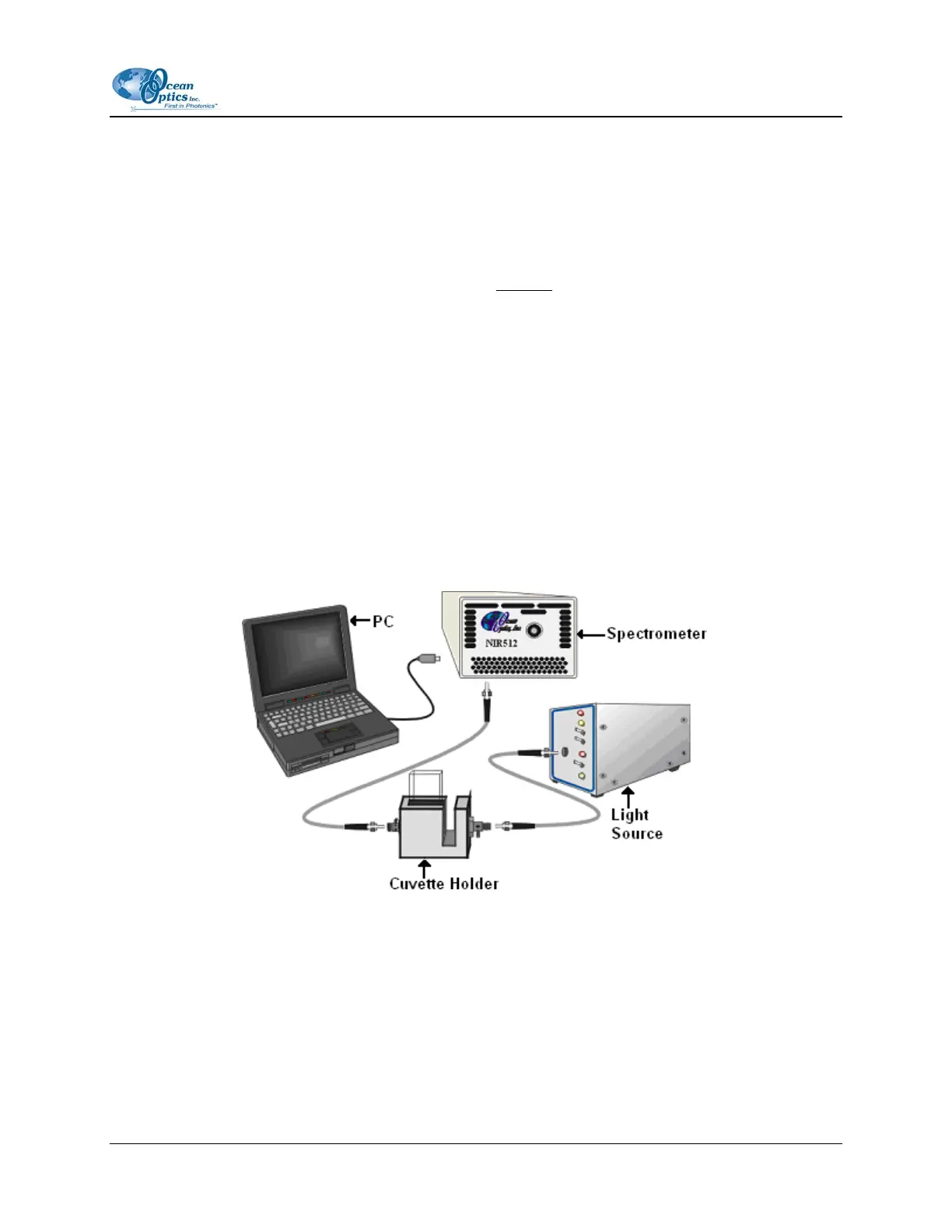
The figure below shows a typical absorbance setup. The light source sends light via an input fiber into a
cuvette in a cuvette holder. The light interacts with the sample. The output fiber carries light from the
sample to the spectrometer, which is connected to the PC.
Absorbance can also be expressed as proportional to the concentration of the substance interacting with
the light, known as Beer’s Law. Common applications include the quantification of chemical
concentrations in aqueous or gaseous samples.
197-00000-512-02-0305 19
 Loading...
Loading...