33
4.1.2. Multichannel Pipettes
Cleaning the outer surface (all models):
Clean any visible dirt with a cleaning solution and a lint-free cloth.
Wipe dry.
Cleaning and maintaining the lower parts of all models
Opening the lower part of a multichannel pipette should only be done by an
authorized Sartorius service provider. Please contact your nearest Sartorius
service provider or distributor.
4.2. Sterilizing
The lower parts of Sartorius electronic pipettes can be sterilized by
autoclaving, with UV or by using disinfectant or decontaminant liquids such
as 70% ethanol, 60% isopropanol, mild detergent, or similar. Always make
sure the materials of the pipette and the disinfectant or decontaminant liquid
are chemically compatible.
Always follow the autoclaving instructions below.
4.2.1. Autoclaving
The lower parts of Sartorius electronic pipettes are autoclavable, excluding
the 1200 µl multichannel model.
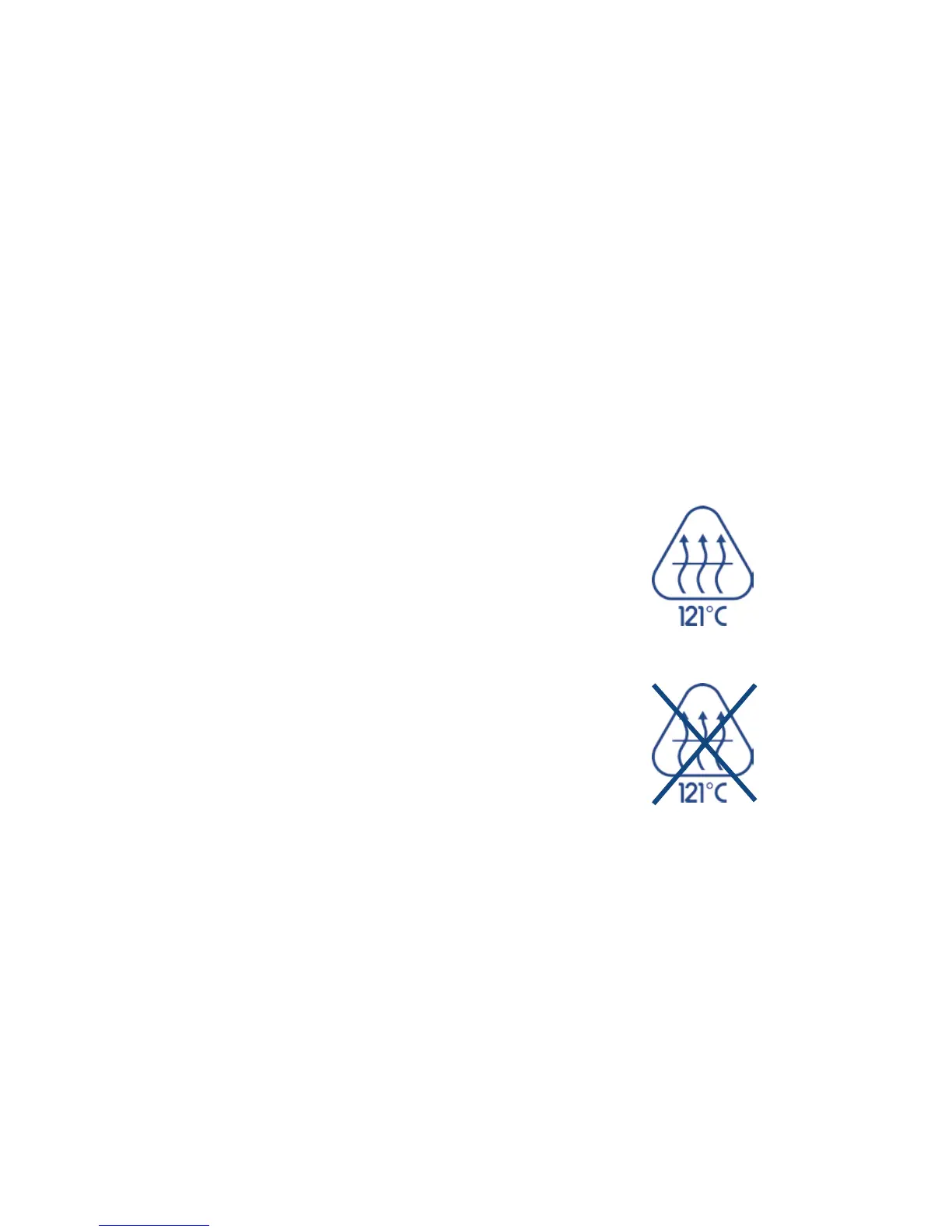
Please see the autoclaving symbol printed on the lower part of your pipette to
ensure the section is autoclavable.
Autoclaving Instructions:
1. Remove the Safe-Cone Filter if attached.
2. Disassemble the lower part:
a. with single channel pipettes, unscrew the tip ejector collar, tip cone
and piston by turning them counter-clockwise. Place these parts in a
sterilisation bag
OR
b. with multichannel pipettes, unscrew the connecting collar counter-
clockwise to remove the tip cone housing, and place it in a sterilisation
bag.
3. Sterilize the parts at 121°C and 1 bar overpressure for 20 minutes.
4. Let the parts cool and dry before reassembling.
4.2.2. UV Sterilization
Sartorius electronic pipettes are made of UV-resistant materials. Sartorius
pipettes tolerate temporary exposure to UV radiation. Take note that
prolonged or frequent exposure to UV radiation may cause yellowing and
brittling of the pipette.
4.3. Performance Testing
It is recommended that the performance of Sartorius pipettes is checked
regularly (e.g. every three months) and always after in-house maintenance.
A regular testing routine should be established by users, taking into
consideration the accuracy requirements of the application, frequency of use,
number of operators using the pipette, nature of the liquid dispensed, and the
acceptable maximum permissible errors (ISO 8655-1).
Performance tests should take place in a draught-free room at 15–30°C, kept
constant within ±0.5°C and with relative humidity above 50%. The pipette,
tips, and test water should have stood in the test room for long enough to
reach equilibrium with the room conditions (at least two hours). Use distilled
or de-ionised water (ISO 3696, grade 3) and an analytical balance that
conforms to ISO 8655-6.
Autoclavable lower part
Not autoclavable lower part

 Loading...
Loading...











