Theory of Operation DF-150E 51
11 Theory of Operation
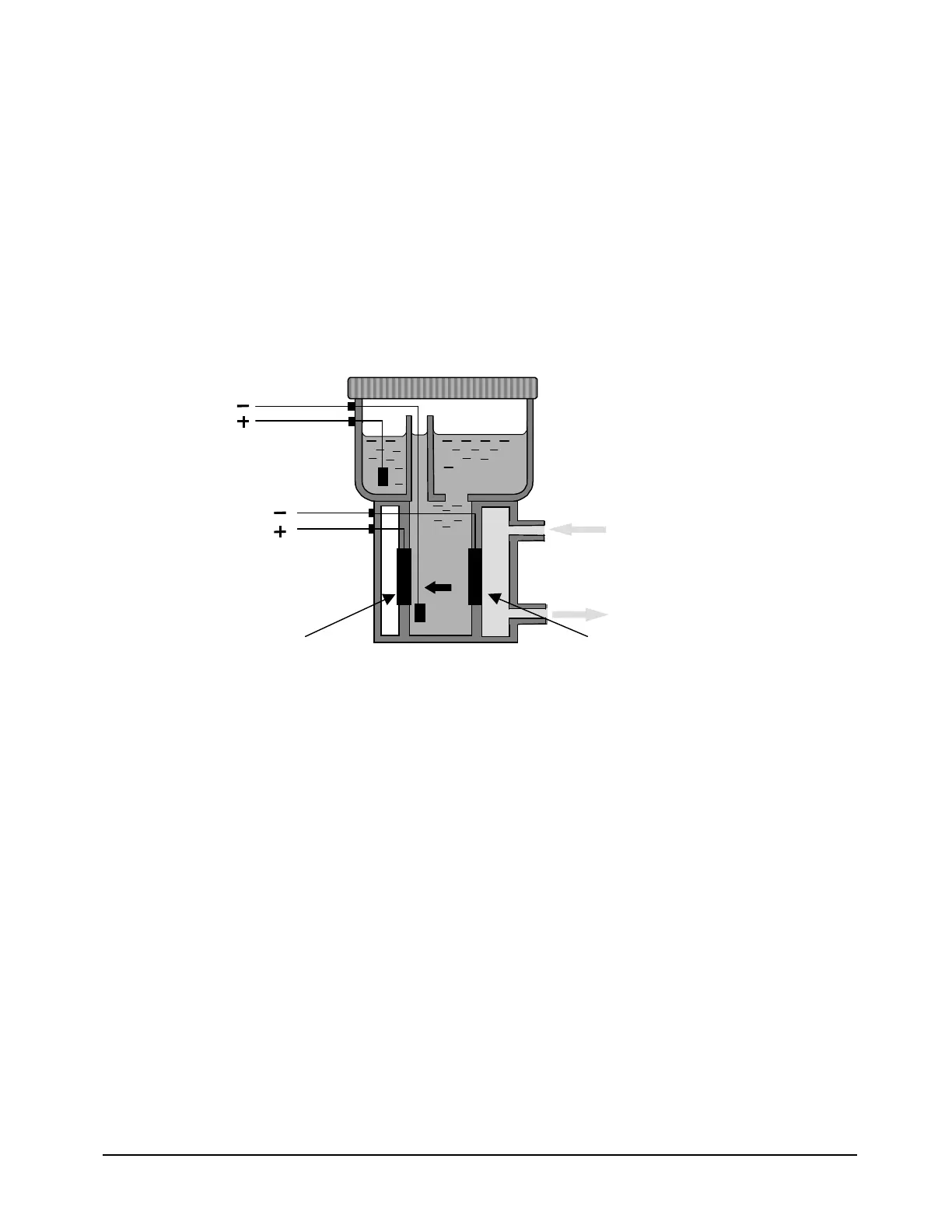
The Servomex Coulometric Sensor uses an ambient temperature oxygen
reaction that is non-depleting. The cell produces a current flow that is
determined by the number of oxygen molecules that are reduced at the
cathode. The sensor reaction is driven by 1.3 Volts applied across the
electrodes. The resulting electron flow is measured as a current that is precisely
proportional to the oxygen concentration in the sample gas.
KOH
4OH
¯
Sample Gas
Cathode
Secondary
Electrodes
Anode
O
2
1.3V Applied
Figure 30: Schematic of DF Series Oxygen Sensor
The cathode reaction uses 4 electrons from the 1.3 volt circuit, 2 water
molecules from the electrolyte, and 1 oxygen molecule from the sample gas to
generate 4 hydroxyl ions which migrate across the reaction chamber to the
anode:
O2 + 2H2O + 4e
-
4OH
-
The anode reaction consumes the 4 hydroxyl ions and delivers 4 electrons to
the circuit, 2 water molecules back to the electrolyte, and vents one oxygen
molecule.
4O H
-
O2 + 2H2O + 4e
-
There is no net change to the electrolyte and no depletion of the sensor or
electrodes.

 Loading...
Loading...