8
IT
EN
DE
FR
ES
PT
1. MODELS
The following basic models may be subject to implementation or change without notice.
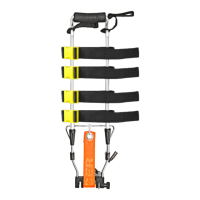
• DAVIS ADULT • DAVIS PAEDIATRIC
2. INTENDED USE
2.1 INTENDED USE AND CLINICAL BENEFITS
Traction systems are devices that limit tissue damage caused by possible bone rubbing by spacing the two halves of a fracture.
2.2 TARGET PATIENTS
There are no particular indications related to the patient group.
The design of the product allows it to be applied to any subject as long as the chosen size of the limb allows for the intended purpose of the device to be achieved.
2.3 PATIENT SELECTION CRITERIA
The expected patients are those for whom traction is necessary.
2.4 CONTRAINDICATIONS AND UNWANTED SIDE EFFECTS
No particular contraindications or side effects are known with relation to use of the device, as long as it is used in accordance with the user manual.
2.5 USERS AND INSTALLERS
The intended users are rescue workers with in-depth knowledge related to the immobilisation and handling of individuals with fractures or suspected fractures.
These devices are not intended for lay people.
Operators must be able to provide the necessary patient care.
The product must be used only by personnel trained in the use of this product and not on other similar products.
The device does not require installation.
3. REFERENCE STANDARDS
REFERENCE DOCUMENT TITLE
EU Regulation 2017/745 EU Regulation on Medical Devices
4. INTRODUCTION
4.1 DEVICE LABELLING AND TRACEABILITY
Each device is provided with a label, placed on the device itself and/or on the packaging, which contains the Manufacturer’s identification data, product, CE marking, serial
number (SN) or lot number (LOT). This must never be removed or covered.
Regulation 2017/745/EU requires manufacturers and distributors of medical devices to keep track of their location. If the device is in a location other than the address to which
it was shipped or sold, or if it was donated, lost, stolen, exported or destroyed, permanently removed from use, or if the device was not delivered directly from Spencer Italia
S.r.l., please register the device at http://service.spencer.it, or inform Customer Service (§ 4.4).
4.2 SYMBOLS
Symbol Meaning Symbol Meaning
Device in compliance with EU Regulation 2017/745
Danger – Indicates a hazardous situation that may result in a situation
directly related to serious injury or death.
Medical device See the instructions for use
Manufacturer Lot number
Date of manufacture Product code
Unique Device Identifier
Caution: Federal law restricts the sale of this device by or on the order of a
licensed professional (US market only)
(01)8057711230006 (11) 200626 (10) 1234567890
Production identification
Alphanumeric code that identifies the production units of the device, composed of:
(01)805771123 company prefix
000 progressive GS1
6 control number
(11)200626 date of production (YYMMDD)
(10)1234567890 lot number
4.3 WARRANTY AND SERVICE
Spencer Italia S.r.l. guarantees that products are free from defects for a period of one year from the date of purchase.
Spencer Customer Service tel. +39 0521 541154, fax +39 0521 541222, service@spencer.it
Warranty and service conditions are available on the website http://support.spencer.it
5. WARNINGS/DANGERS
Product features
Use of the product for any purpose other than that described in the User Manual is prohibited.
• The product must not be tampered with or modified without the manufacturer’s authorisation.
• Avoid contact with sharp or abrasive objects.
• Operating temperature: from -5°C to + 50°C.
• Storage temperature: -10°C to +60°C.
General warnings for medical devices
• Do not use if the device or parts of it are punctured, torn, frayed, or excessively worn.
• Do not alter or modify the device arbitrarily, as doing so could result in unpredictable operation and damage to the patient or rescuers and shall void the manufacturer’s
warranty and release the manufacturer from all liability.
• Participate in safety checks on products placed on the market, transmitting information regarding product risks to the Manufacturer as well as to the Competent Authorities
 Loading...
Loading...