9
IT
EN
DE
FR
ES
PT
for their respective actions.
• With reference to EU Regulation 2017/745, please note that public or private operators who, when exercising their activity, detect an incident involving a medical product
are required to notify the Ministry of Health, within the terms and in the manner established by one or more ministerial decrees, and notify the Manufacturer. Public or
private health care professionals are required to notify the Manufacturer of any other incident that may allow the adoption of measures to ensure the protection and health
of patients and users.
6. SPECIFIC WARNINGS
To use the traction system, you must also have read, understood and carefully follow all the instructions in the user manual.
• Perform traction simulations with dummies before putting the device into service.
• Improper use or use by untrained operators may result in injury or permanent disability. Always follow the procedures and protocols approved by the relevant
Emergency Medical Service.
• To preserve the life of the device, protect it as much as possible from UV rays and adverse weather conditions.
• If the product is found to be malfunctioning, immediately use a similar device to ensure continuity of ongoing operations. Non-compliant devices must be taken out of
service.
• Qualified personnel must be present during use of the device.
• The traction system should be applied by at least two trained rescuers who have good manual dexterity and common sense and are trained in emergency rescue procedures.
• Before applying the traction system, appropriate primary medical evaluations should be performed to assess both the appropriateness of applying the device and any
additional actions that should be taken in relation to the patient’s injuries.
• The device must not be exposed, much less come into contact with thermal sources of combustion or flammable agents.
• Only use the device as described in this manual.
• Always check the conditions of all parts before use.
7. RESIDUAL RISK
No residual risks are identified, i.e. risks that could arise despite compliance with all the warnings in this user manual.
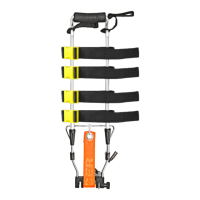
8. TECHNICAL DATA AND COMPONENTS
DAVIS/DAVIS PAEDIATRIC
PARTS DESCRIPTION AND MATERIALS
1 Gear wheel Made of aluminium, allows locking of the traction mechanism
2 Traction mechanism support
Made of nylon, this is the structure on which the gear wheel, the pin around which the belt is wound, and the knob
with which traction is performed are installed.
3 Traction belt
Made of nylon and equipped with an eyelet to which a hook is attached; this is the element that transmits traction
to the limb.
4 Locking ring Made of plastic material, these allow the removable portion of the frame to be locked in the desired position
5 Ankle brace Made of padded PVC and nylon, this is applied to the patient's ankle allowing traction of the limb
6 Support bands
Made of nylon, these support the limb and, thanks to the closing straps with quick-release buckles, allow the device
to be attached to the limb
7 Ischial support Made of polyurethane, this is the supporting part of the end of the femur
8 Fixed frame Made of aluminium, this is the fixed part of the telescopic frame
9 Mobile
Made of aluminium, this is the telescopic part of the frame that allows the device to be extended to suit the height
of the specific patient
10 Lock pin Made of aluminium, this is a pin that locks the support in the open position
11 Support
Made of aluminium, when open it keeps the device slightly inclined and allows the limb to be moved away from the
ground so that it does not touch the heel
12 Traction knob Made of plastic, when rotated this turns the pin on which the belt is wound, generating traction
Characteristics David adult Davis paediatric
Minimum length(1) 890 ± 10 mm 810 ± 10 mm
Maximum length(1) 1350 ± 10 mm 1170 ± 10 mm
Width 210 mm 185 mm
Base width 230 mm 225 mm
Width of leg support area 165 mm 135 mm
Traction belt length From 0 to 500 mm From 0 to 500 mm
Inclination 10° (variable depending on the extension) 10° (variable depending onthe extension)
Materials Steel,Al, Nylon Steel,Al, Nylon
Weight 1,78 ± 0,1 kg 1,58 ± 0,1 kg
Bag dimensions 990X330 mm 920x300 mm
Bag weight 520 g 320 g
(1) The limb must be approximately 15 cm shorter than the indicated measurements
Sizes subject to ± 10 mm tolerances.
 Loading...
Loading...