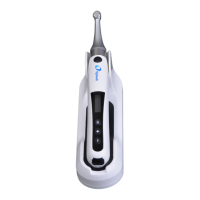
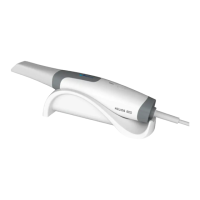
Use only approved autoclave devices
according to EN 13060 or EN 285.
Use a validated sterilization procedure
according to ISO 17665.
Respect the maintenance procedure of
the autoclave device given by the
manufacturer.
Use only this recommended sterilization
procedure.
Control the efficiency (packaging
integrity, no humidity, color change of
sterilization indicators, physicochemical
integrators, digital records of cycles
parameters).
The sterilization procedure must comply
with ISO 17665.
Waiting for cooling before touching.
Sterility cannot be guaranteed if
packaging is open, damaged or wet.
Check the packaging before using it
(packaging integrity, no humidity and
validity period).
 Loading...
Loading...