3 Components
ers2 – 1-Channel ECG Telemetry System 23
201000401000 / Rev 04
3.1.1 Unique Device Identification (UDI)
The Unique Device Identification (UDI) system is a globally harmonized system for the identifica-
tion of medical devices in machine-readable form.
The following UDI information for each component of the ECG system is saved in the form of a bar
code on the respective type plates. This bar code can be read with an appropriate reading device
(e.g., a cell phone). The code will then be displayed on the cell phone:
Code structure:
1-channel transmitter, receiver
01
xxxxxxxxxxxxxx 11 xxxxxx 21 xxxxxxxxxx
Adapter
01
xxxxxxxxxxxxxx 10 xxxxxxxxxxxxx
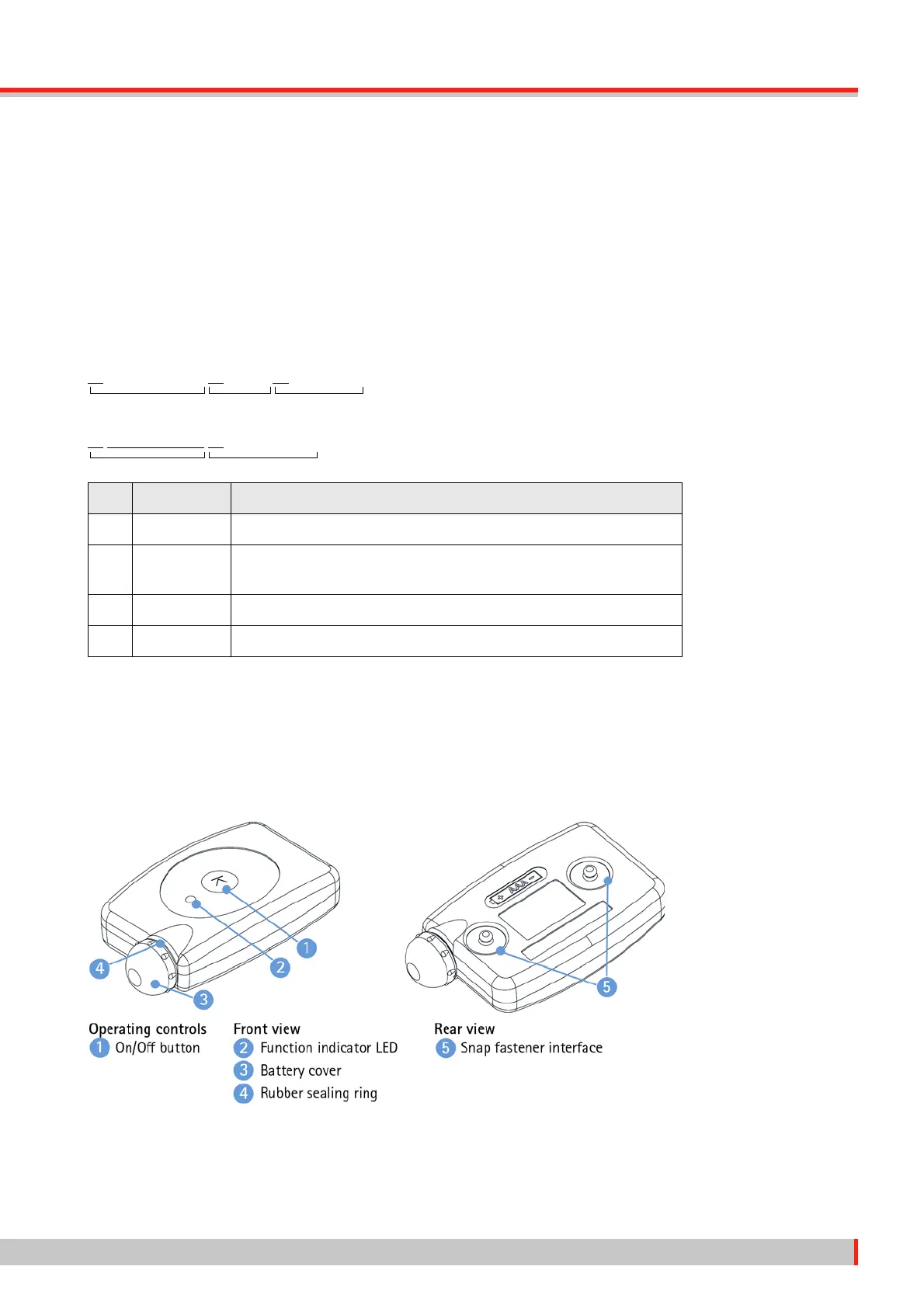
3.2 1-Channel Transmitter
Via the applied part, the 1-channel transmitter acquires a single-channel, bipolar raw ECG signal
and sends it to a medical application software or other ME equipment by radio transmission. The
1-channel transmitter is a type CF, defibrillation-proof applied part.
ID Name Description
01 UDI basic DI 14-digit primary identification of the device model (all components)
11 UDI PI 6-digit date of manufacture, YYMMDD format (1-channel transmitter,
receiver)
21 UDI PI 10-digit serial number (1-channel transmitter, receiver)
10 UDI PI 13-digit lot or batch number (adapter)
 Loading...
Loading...