D
IRECTION 5813707-100, REVISION 2 VENUE GO™ SERVICE MANUAL
Chapter 1 - Introduction 1-25
PRELIMINARY
Batch code
Indicates the manufacturer’s batch code so that the
batch or lot can be identified.
Rear panel or
e-Label
ISO 15223-1 Ref. 5.1.5
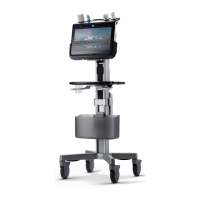
Unique Device Identification (UDI) Label
Every system has a unique marking for
identification, the Unique Device Identification
(UDI) Label. The UDI label consists of a series of
alpha-numeric characters and barcode which
uniquely identify the Venue Go system as a
medical device manufactured by General Electric.
Scan or enter the UDI information into the patient
health record as required by country-specific laws.
Rear panel or
e-Label
Do not put the battery in fire. Battery pack
Do not disassemble or mistreat the battery. Battery pack
Non-Ionizing Electromagnetic Radiation Wireless
LAN
Rear panel
Table 1-5 Label Icons and Symbols - Description and Location (Continued)
Label Name Description Location Source
 Loading...
Loading...