6
Infection Control / Intended Use
2. Infection Control
WARNING: Before fi tting cuffs to the patient, evaluate the cross
contamination risk. For medium/high risk situations, where
the patient has a known infection or skin is not intact, use an
infection control barrier sleeve with aseptic technique.
Infection control barrier sleeves are available as an accessory, Part No. ACC-
VAS-016.
Refer to Section 12.4 for sleeve and cuff fi tting instructions.
Refer to Section 14.2 for care and cleaning instructions.
WARNING: Infection control barrier sleeves are single use devices
and must not be re-used. They must be disposed of as infectious
clinical waste.
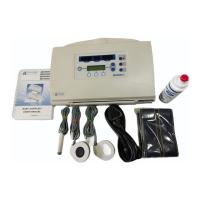
3. Intended Use
It is intended to be used on all patients considered to be at risk of having, or
developing peripheral arterial disease (PAD).
Dopplex Ability is intended for the rapid measurement of ankle-brachial pressure
index (ABPI) or ankle-brachial index (ABI) in adults and pulse volume recording
(PVR) / volume plethysmography.
Ability is suitable for use in woundcare assessment, for assessing symptomatic
PAD, and as a screening device for PAD.
It may also be used on patients with venous or arterial ulcers prior to the
application of compression therapy.
Dopplex Ability can be used on patients with unilateral lower limb amputation.
3.1 User Requirements
Dopplex Ability is intended for use only by a suitably qualifi ed healthcare
professional. If an ABI test is undertaken by a Healthcare Support Worker,
then patient selection and assessment of results must be performed by a
qualifi ed clinician in conjunction with a clinical assessment.
It is recommended that all users familiarise themselves with the information
provided in this user manual and in the enclosed Training Pack before using this
product.

 Loading...
Loading...