MAINTENANCE
105
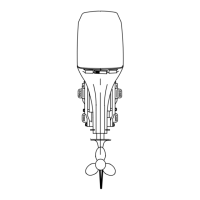
Corrosion and Corrosion Protection
Whenever two or more dissimilar metals (like those found on the
sterndrive) are submerged in a conductive solution, such as
saltwater, polluted water, or water with a high mineral content, a
chemical reaction takes place causing electrical current to flow
between metals. The electrical current flow causes the metal that
is most chemically active, or anodic, to erode. This is known as
galvanic corrosion and, if not controlled, it will in time cause the
need for replacement of power package components exposed to
water.
Refer to the Quicksilver booklet, Everything You Need to Know
About Marine Corrosion for more corrosion information.
NOTICE
Using magnesium anodes in salt water causes an
electrochemical reaction on the metal surface of the drive,
resulting in corrosion damage from the paint blistering and
peeling off the drive. Use magnesium anodes in fresh water
only.
 Loading...
Loading...