10 Calculation Methods 10-1
10 C
aculation Methods
10.1 Reaction Type
The system provides three reaction types for measurement: Endpoint, Fixed-time and
Kinetic.
10.1.1 Endpoint
The endpoint or, more correctly, equilibrium method, is most ideal. The reaction reaches
equilibrium after a period of time. Since the equilibrium constant is very large, it can be
considered that all substrates (analytes) have changed into products, and absorbance of
the reaction liquid does not change any more. The absorbance change is directly
proportional to the analytes concentration.
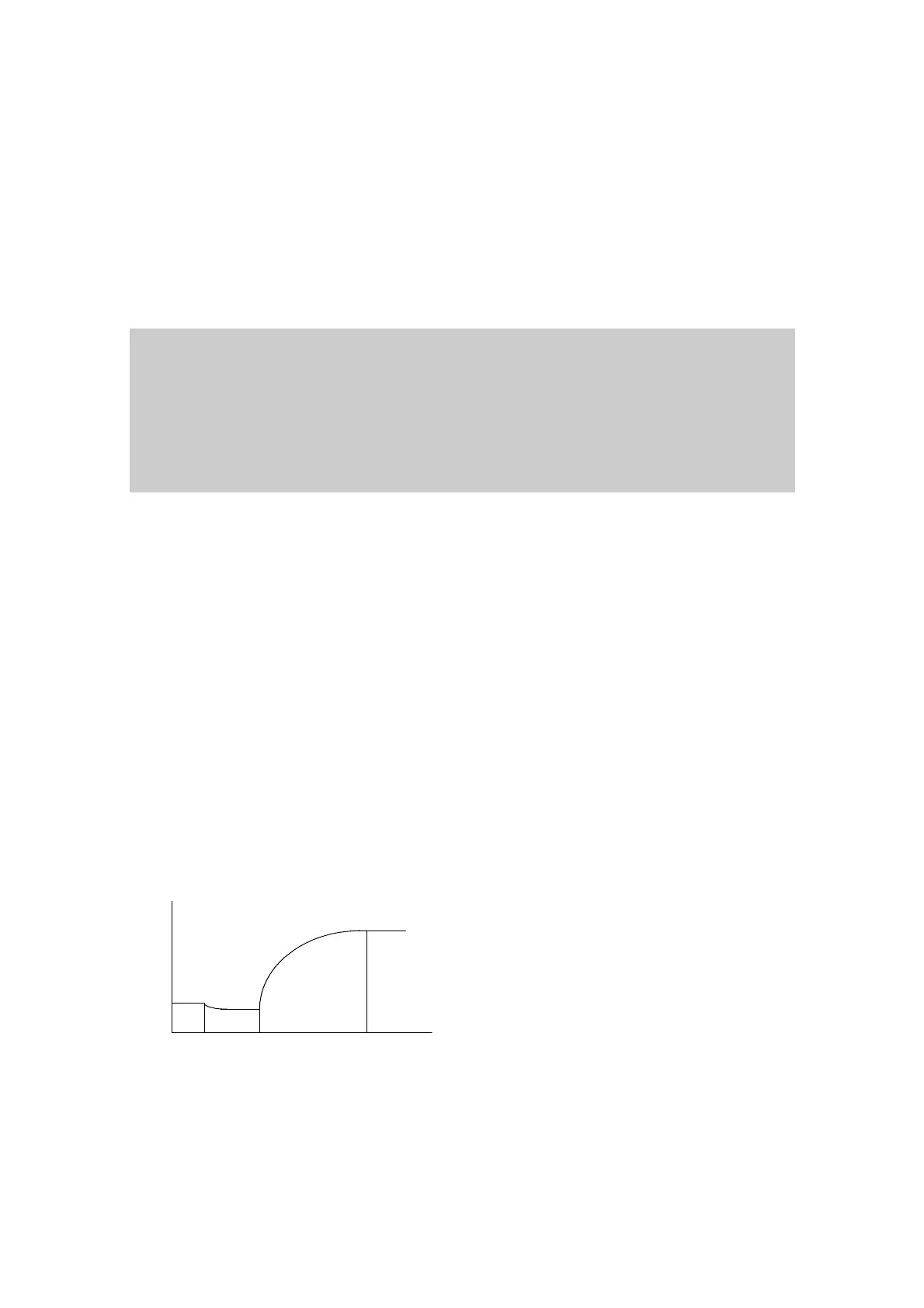
Figure 10-1 Single-reagent Endpoint Reaction Curve
A
t
t1
t2
t3
As shown in Figure 10-1,
1
t is the time when the reagent is added, and
2
t is the time
when the sample is added. The reaction starts when they are mixed. At
3
t
the reaction
reaches equilibrium and the absorbance reading is taken. The reaction period is
2
t to
3
t
.
 Loading...
Loading...