10 Calculation Methods
10-2
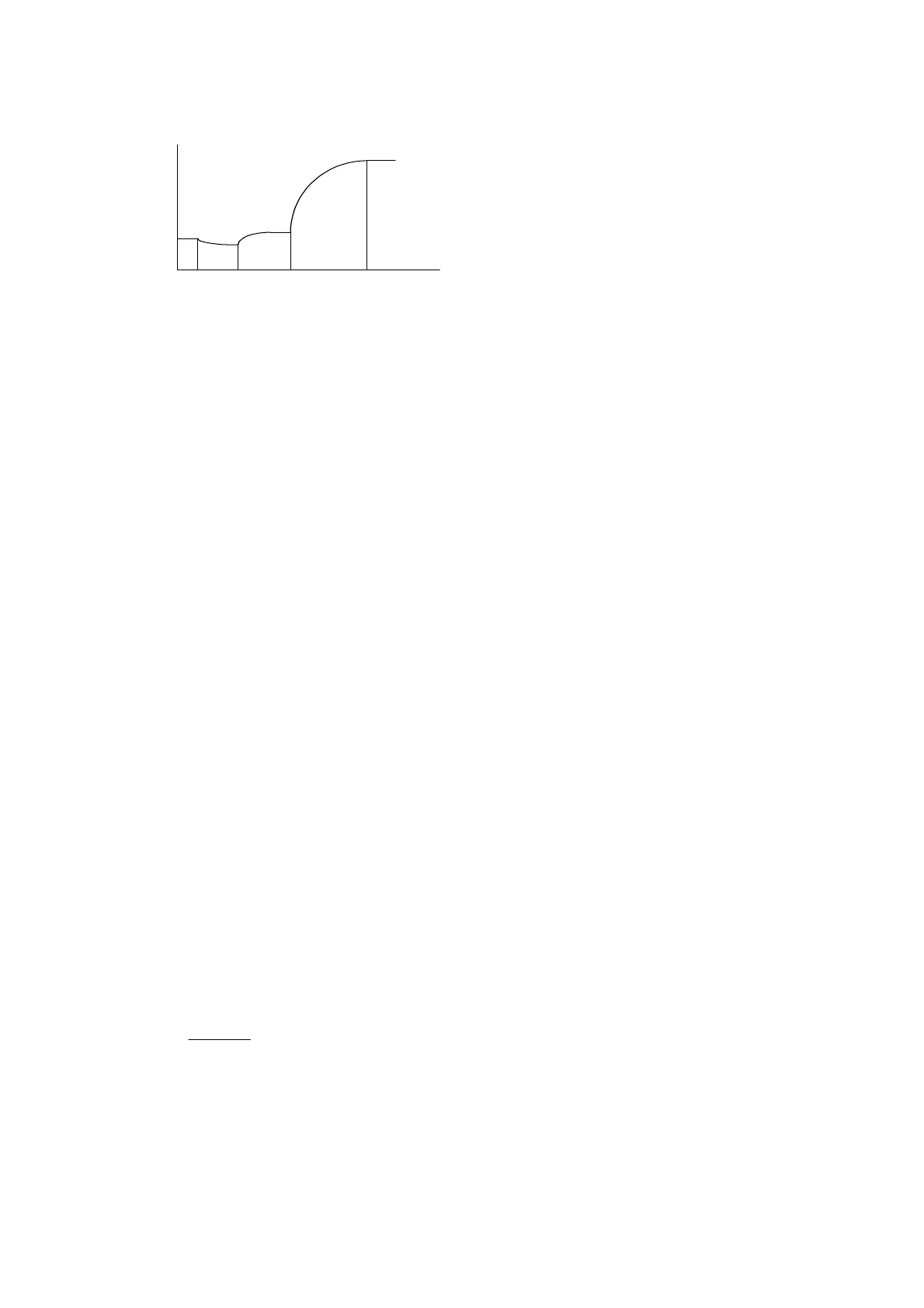
Figure 10-2 Double-reagent Endpoint Reaction Curve
A
tt1 t2 t3 t4
As shown in Figure 10-2 ,
1
t is the time when the first reagent is added, and
2
t is the
time when the sample is added, incubation starts when they are mixed.
3
t
is the time
when the second reagent is added, then the reaction starts when they are mixed. At
4
t the
reaction reaches equilibrium and the absorbance reading is taken.
2
t to
3
t
is the
incubation period and
3
t
to
4
t is the reaction period.
The endpoint reaction is largely insensitive to minor changes in such condition changes as
amount of enzyme, pH and temperature, provided the changes are not significant enough
to affect the reaction time.
10.1.2 Fixed-Time
For the fixed-time reaction method (namely, first-order kinetic method or initial rate
method), the reaction velocity (v), within a specific period, is directly proportional to the
substrate concentration [S], namely, v=k[S]. As the substrate is consumed continuously,
the reaction velocity becomes smaller and smaller, and so does the change rate of the
absorbance. It takes much time for such a reaction to reach equilibrium. Theoretically, the
absorbance reading can be taken at any time. The reaction can, however, become steady
only after a delay because it is complicated at the beginning and there are miscellaneous
reactions due to the complex serum compositions. For any first order reaction, the
substrate concentration [S] at a given time after the start of the reaction is given by the
following:
kt
eSS
−
×=
0
Where,
[S
0
] - initial substrate concentration,
e - base of the natural log,
k - rate constant.
The change in substrate concentration ∆[S] over a fixed-time interval,
1
t to
2
t , is related
to [S
0
] by the following equation:
S
S
ktkt
21
][
][
0
−−
−
∆−
=
That is, within a fixed time interval, the change in substrate concentration is directly
proportional to its initial concentration. This is the general property of first order reactions.
Within this interval, absorbance change is directly proportional to the analytes
concentration.
 Loading...
Loading...