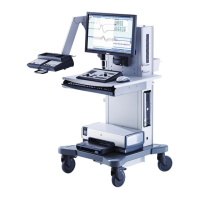
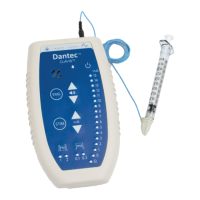
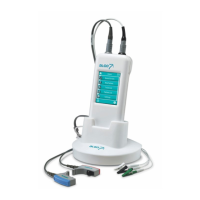
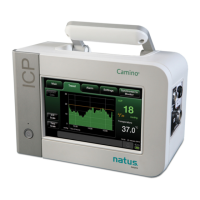
Dantec Keypoint Focus
11
Adhere to the following recommendations for safe operation of the device:
When connecting medical equipment being supplied from an outlet located in a non-
medically used room, or when connecting non-medical electrical equipment to this
device, please pay attention to the requirements of IEC 60601-1, Safety Requirements
for medical electrical systems, cf. the text on IEC 60601-1, further below in this section.
When the device is connected to its mains supply, connectors may be live, and any
opening of covers or removal of parts possible only with the aid of a tool is likely to
expose live parts.
The device must be disconnected from all voltage sources before being opened for any
adjustment, replacement, maintenance or repair.
Service must be referred to Natus authorized service personnel, except for such works
described in this manual as being performed by the operator.
Make sure that only fuses with the required rated current and of the specified type are
used for replacement. The use of makeshift fuses and the short-circuiting of fuse
holders are prohibited.
Where more than one piece of equipment is connected to the patient, attention must
be paid to the summation of patient leakage currents.
Whenever it is likely that the protection has been impaired, the device shall be made
inoperative and be secured against any unintended operation.
Call qualified service personnel to conduct at least a functional test and a safety check
that should include the following:
-Insulation test;
-Ground continuity test; and
-Leakage current test, according to IEC 60601-1.
The protection is likely to be impaired if, for example, the device:
-Shows visible damage;
-Fails to perform the intended function(s);
-Has been subject to severe transport stresses.
 Loading...
Loading...