100c User Manual
44
ch.7
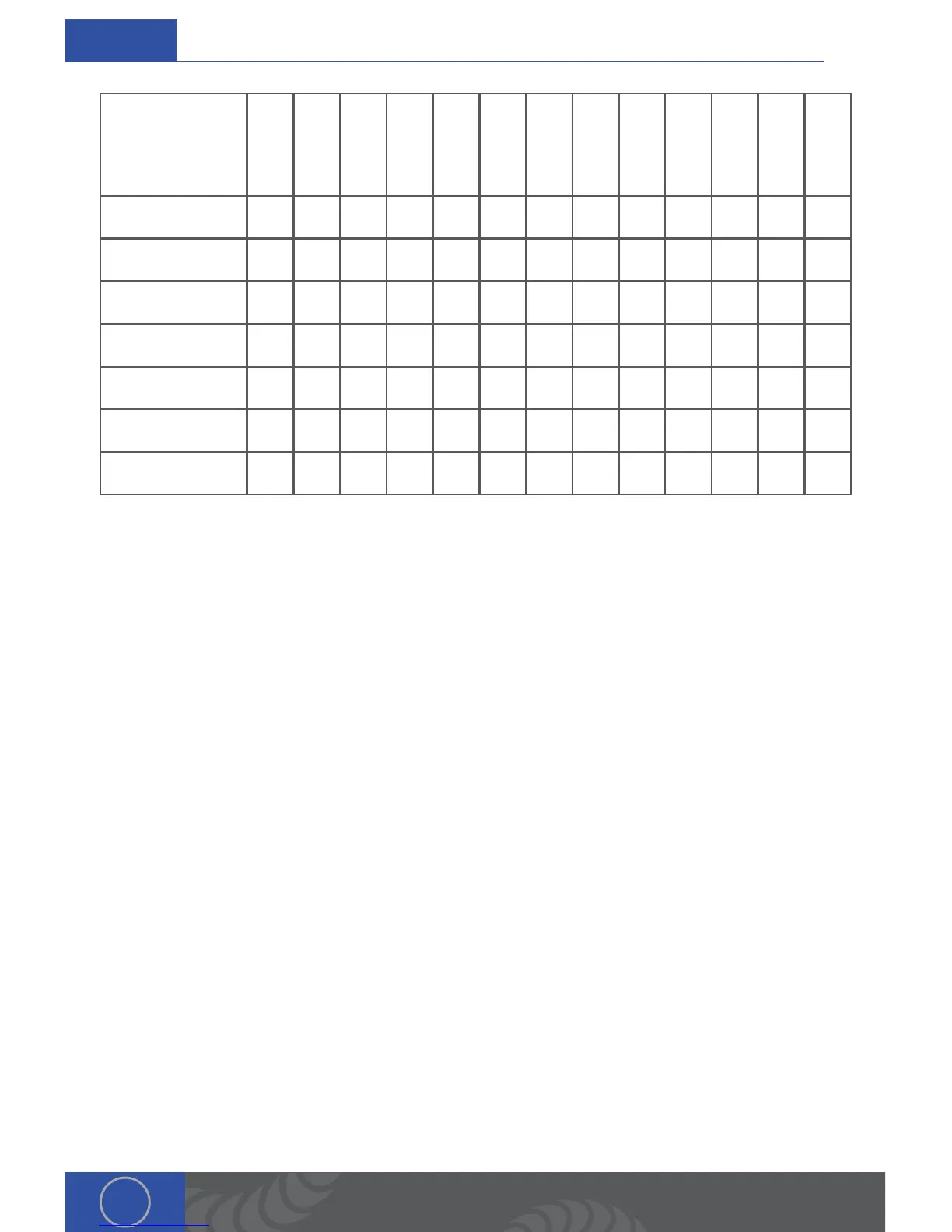
Zinc
Aluminum
Silver
Gold
Copper
Palladium*
Cobalt
Chrome*
Stainless
304*
Carbon Steel
Titanium
Platinum
Niobium
Tungsten
Melting Point
420 660 962 1064 1083 1200 1300 1450 1500 1660 1772 2468 3410
Boiling Point
607 2467 2212 3080 2567 3100 2800 3000 3000 3287 3827 4742 5660
Specific Heat
388 900 237 129 385 244 10 500 500 523 129 268 133
Electrical Resistivity
6 2.7 1.6 2.2 10.6 10.8 475 70 60 54 10.6 16 5.4
Density
7.1 2.7 10.5 19.3 9 11 8.3 7.9 7.8 4.5 21.5 8.6 19.3
ermal Expansion
31 23.5 19.1 14.1 17 11 10 18 12 8.9 9 7.2 4.5
ermal Conductivity
116 237 429 318 401 71 100 16.3 50 22 71.6 54 173
*Some Values may be approximate
Melting Point: e temperature at which the metal will begin to melt. e molten metal of the
weld pool will be at this temperature during the welding process.
Boiling Point: If enough energy is added to the weld joint (and heat is removed slowly by the
surrounding solid metal) the weld puddle can begin to boil. Liquid metal will be turned into
gaseous metal.
Specific Heat: e energy required to raise the temperature of the metal (per unit mass). ink
of this number as how much metal will melt for a given weld energy (melting point also is
important). A larger specific heat means more energy is required to melt the metal.
Electrical Resistivity: is number represents the resistance to the flow of electrons in a
metal. is property is especially important during a resistance or “tack” weld. e more
resistive the metal is the more easily it will resistance weld (e.g. stainless steels), the
smaller this number is the more difficult it will be to weld the material (e.g. silver), especially
in “tack” mode.
Density: how much of the metal (atoms / mass) is in a given volume of space. is property
will also influence how large the weld spot is for a given metal. All other things being equal,
a lower density metal will have a larger weld spot than a higher density metal for the same
weld energy.
ermal Expansion: When a metal is heated it will expand, or elongate slightly. In some
situations, especially during resistance welding, metal can expand quickly and spill out of
the weld joint.
ermal Conductivity: is is a measure of how fast the metal conducts heat. Metals that
are good conductors of heat (e.g. copper) will dispel the heat away from the weld location
quickly during the welding process. is action reduces the size of the weld spot. Metals
that are poor conductors of heat (e.g. titanium) are slow to conduct heat away from the weld
location and the weld energy has a greater affect on the weld size, etc.
 Loading...
Loading...