Introduction
English 1
Introduction
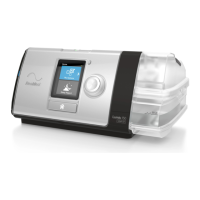
The Astral device provides mechanical ventilation to both ventilation dependent and non-dependent
patients. It delivers pressure and volume ventilation through either a valve or leak circuit, and is
compatible with a range of accessories to support specific use cases.
The information in this guide applies to both the Astral 100 and the Astral 150 devices. Where
information applies to only one of these devices, that device will be specified.
Note: Some features may not be available on your device.
This User Guide is for a patient or carer user, and does not contain all the information provided in the
Clinical Guide.
WARNING
Read the entire manual before using the Astral device.
• Use the Astral device only as directed by a physician or healthcare provider.
• Use the Astral device only for the intended use as described in this manual. Advice contained
in this manual does not supersede instructions given by the prescribing physician.
• Install and configure the Astral device in accordance with the instructions provided in this
guide.
CAUTION
In the US, Federal law restricts this device to sale by or on the order of a physician.
Indications for use
The Astral 100/150 provides continuous or intermittent ventilatory support for patients weighing more
than 5 kg who require mechanical ventilation. The Astral device is intended to be used in home,
institution/hospital and portable applications for both invasive and non-invasive ventilation.
CAUTION
The Astral device is not intended for use as an emergency transport ventilator.
Contraindications
The Astral device is contraindicated in patients with the following pre-existing conditions:
• pneumothorax or pneumomediastinum
• pathologically low blood pressure, particularly if associated with intravascular volume depletion
• cerebrospinal fluid leak, recent cranial surgery or trauma
• severe bullous lung disease
• dehydration.
WARNING
AutoEPAP is contraindicated when using an invasive interface.

 Loading...
Loading...