Oxygen Demand, Chemical
Oxygen Demand, Chemical
Page 2 of 8
OxygenCOD_Other_Other_MD3_Eng_Ody.fm
Calculating the Multiplication Factor
All dilutions require that the ratio of sample to sulfuric acid remain at 9:1. For
other dilutions that are not listed in Table 1 , add the sample volume and the
deionized water and divide by the sample volume to obtain the multiplication
factor.
For best results, use 0.5 mL or more of sample for diluting. If sample values
exceed 18,000 mg/L COD, use a separate sample dilution before performing the
sample chloride removal procedure.
Example: Dilute the sample to a range of 90–4500 mg/L COD.
Sample Volume (2.0 mL) + Deionized water (7.0 mL) = Total Volume (9.0 mL)
Standard test range is 50 to 1000 mg/L COD.
Example test range = 4.5(50) to 4.5(1000) = 225 to 4500 mg/L COD
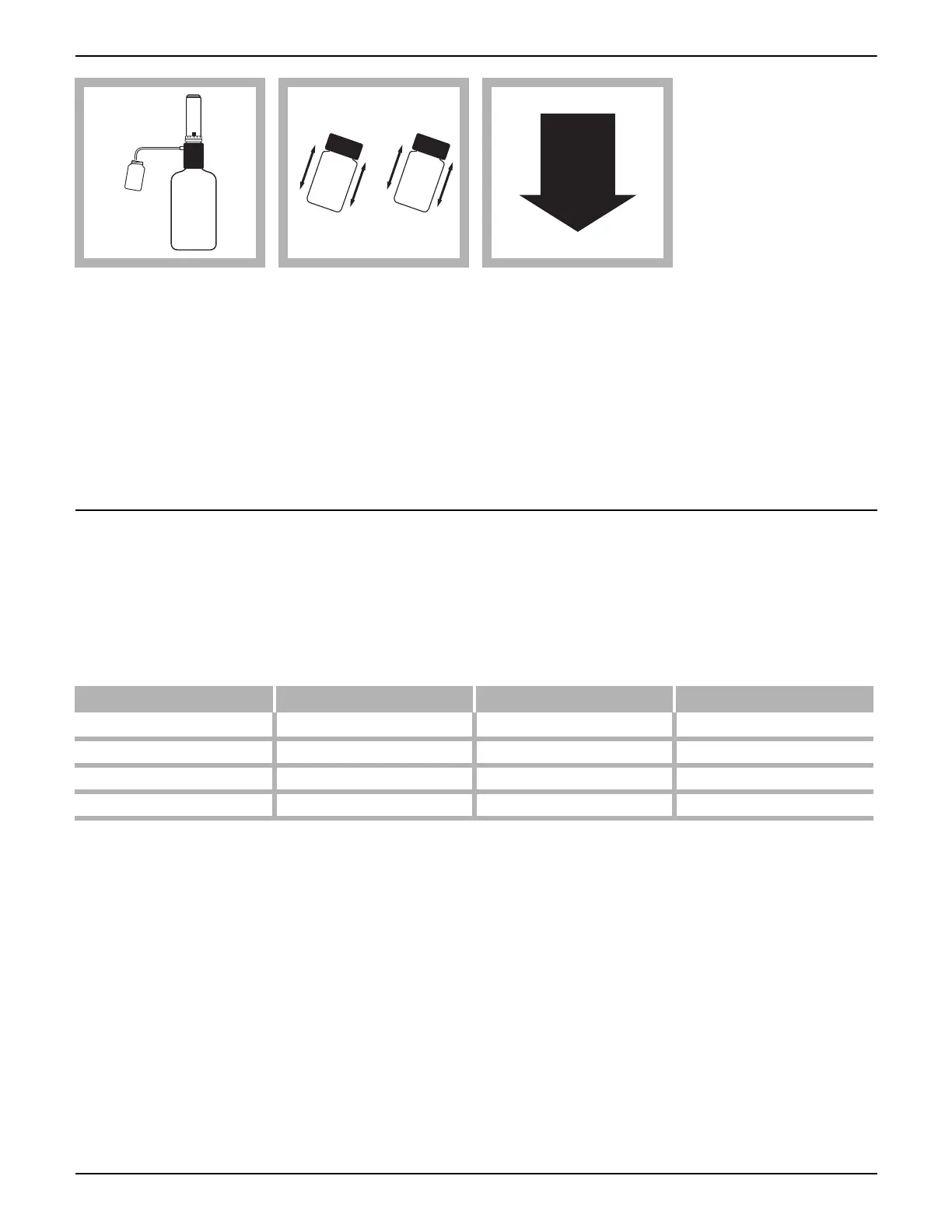
4. Using an automatic
dispenser (Cat. No.
25631-37) or TenSette
®
Pipet, add 1.0 mL of
concentrated sulfuric
acid to both the sample
cell and the blank.
5. Cap the cells tightly
and invert several times.
The solution will become
hot. Cool to room
temperature before
proceeding.
Acidified samples are
stable for several months
when refrigerated at 4 °C.
6. Proceed to Tips and
Techniques for Using the
Vacuum Pretreatment
Device on page 3.
See
Below
Table 1
Sample (mL) Deionized Water (mL) Range (mg/L COD) Multiplication Factor
6.0 3.0 30–1500 1.5
3.0 6.0 60–3000 3
1.0 8.0 180–9000 9
0.5 8.5 360–18,000 18
Multiplication Factor
Total Volume
Sample Volume
------------------------------------------
9.0 mL
2.0 mL
------------------ 4.5===

 Loading...
Loading...