Invacare®EtudePlus
WARNING!
Riskofmalfunctionduetoelectromagnetic
interference
Donotusethisbedadjacenttoorstackedwith
otherelectricalequipment,thanspeciedinthe
following,asitcouldresultinimproperoperation.
Ifsuchuseisnecessary,thebedandtheother
equipmentmustbecloselyobservedtoverify
thattheyareoperatingnormally.
ThisbedcanbeusedtogetherwithInvacare
approvedaccessoriesandmedicalelectrical
equipmentconnectedtotheheart(intracardially)
orbloodvessels(intravascularly)providedthat
followingpointsarerespected:
–Medicalelectricalequipmentshouldnotbe
xedonthebed’smetallicaccessoriessuchas
siderails,liftingpole,driprod,bedends,etc.
–Themedicalelectricalequipmentpowersupply
cordshouldbekeptclearoftheaccessoriesor
anyothermovingpartsofthebed.
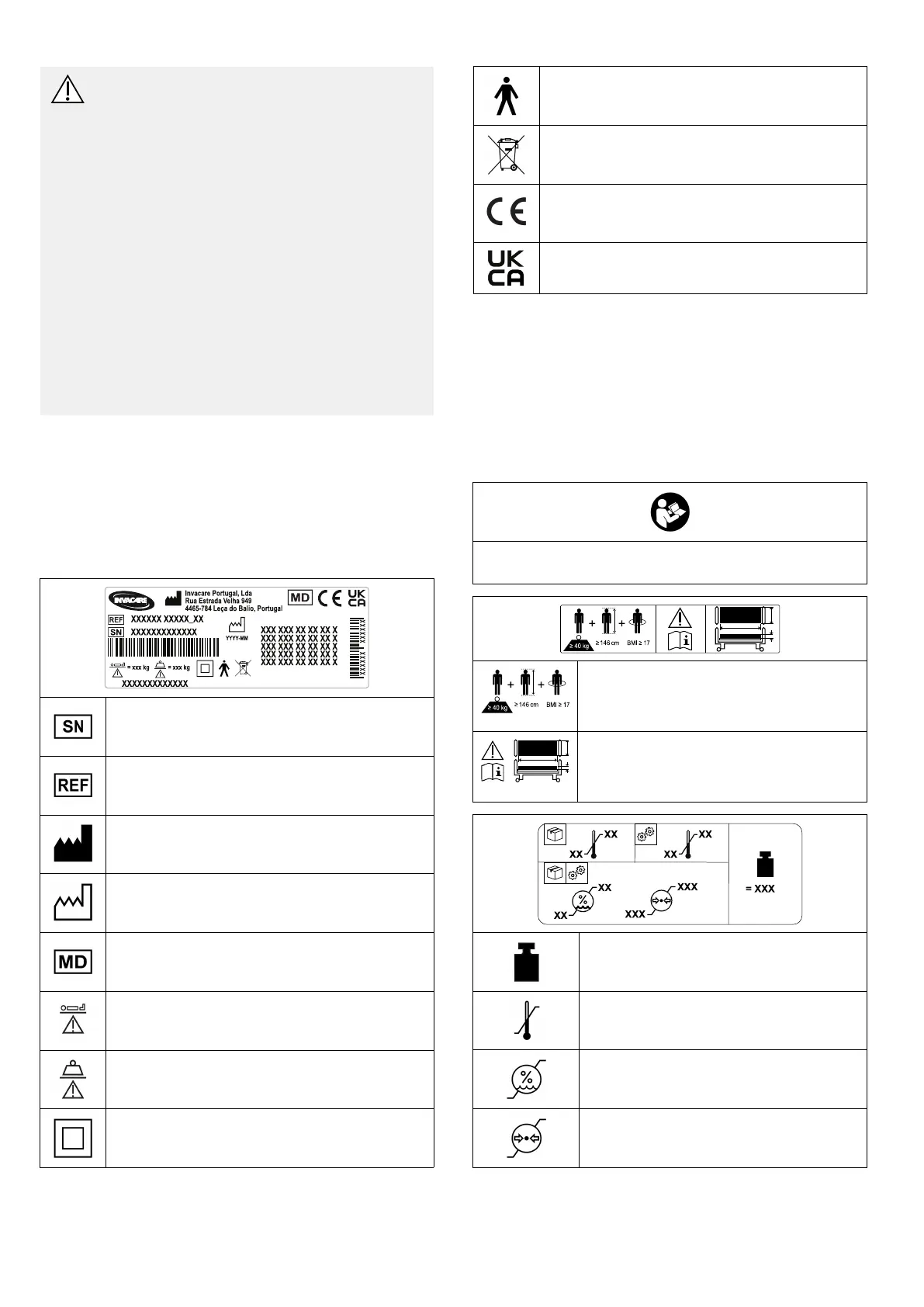
2.4Labelsandsymbolsontheproduct
2.4.1Identicationlabel
Theidenticationlabelisplacedontheframeofthebedand
containsthemainproductinformation,includingtechnical
data.
YYYY-MM
Invacare Portugal, Lda
Rua Estrada Velha 949
4465-784 Leça do Balio, Portugal
= xxx kg
XXXXXXXXXXXXX
XXX XXX XX XX XX X
XXX XXX XX XX XX X
XXX XXX XX XX XX X
XXX XXX XX XX XX X
XXX XXX XX XX XX X
Serialnumber
Referencenumber
Manufacturer
Dateofmanufacture
Medicaldevice
Max.userweight
Max.safeworkingload
CLASSIIequipment
TypeBappliedpart
WEEEconform
EuropeanConformity
UKConformityAssessed
Abbreviationsfortechnicaldata:
•Iin=IncomingCurrent
•Uin=IncomingVoltage
•Int.=Intermittence
•AC=AlternatingCurrent
•Max=maximum
•min=minutes
Formoreinformationabouttechnicaldata,referto9
TechnicalData,page23.
2.4.2OtherLabels
Readtheusermanualbeforeusingthisproductandfollow
allinstructionsforsafetyanduse.
Denitionofmin.weight,min.heightand
min.bodymassindexofanadultuser.
SeeIntendedUse.
Refertouserdocumentationforthe
correctmattressmeasures.
See9TechnicalData,page23.
Totalweightoftheproductwiththe
maximumsafeworkingloadapplied
Temperaturelimit
Humiditylimitation
Atmosphericpressurelimitation
860128467-B
 Loading...
Loading...











