User Guide CGM
Attachment D Technical Information 76
Attachment D: Technical Information
D.1. Device Performance Characteristics
Summary
Sinocare assessed iCan i3 CGM performance in a clinical study with 60
adults (18 years and older) participants. The participants all had type 1
or type 2 diabetes.
Participants wore devices for up to 15 days on their abdomen.
Each participant attended least one of clinical session during the
beginning (Day 2), middle (Day 7-9), or end (Day 15) of the 15 days
wear period to have their venous blood glucose measured every
15minutes with a laboratory reference method, the Yellow Springs
Instrument 2900D Biochemistry Analyzer.
The iCan i3 CGM device was compared to the laboratory reference
method to evaluate accuracy in participants aged 18 years and older.
Accuracy
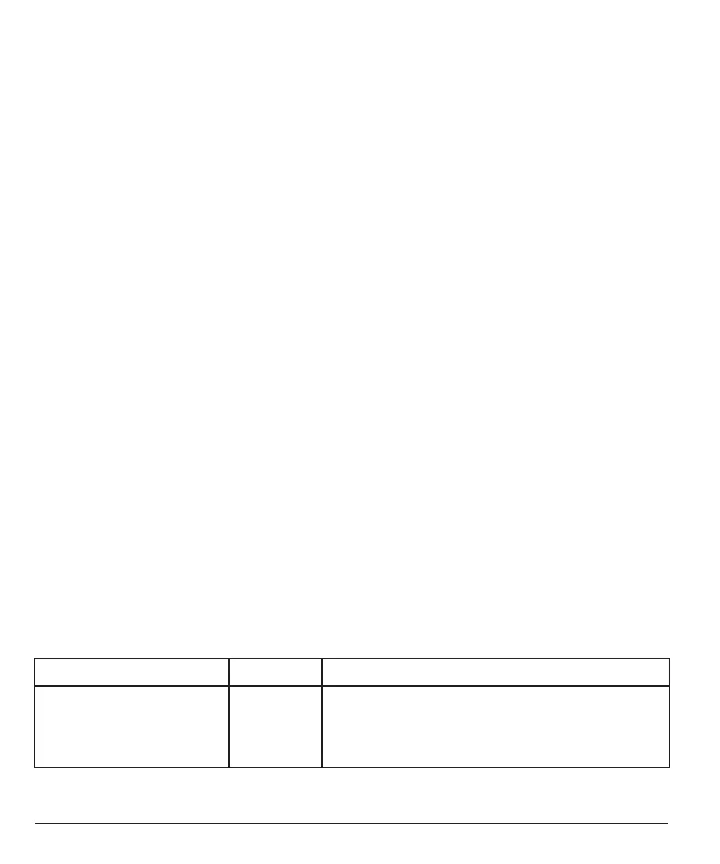
The accuracy of the iCan i3 CGM is shown in the table below.
Mean absolute relative dierence (MARD) is a measure that shows
on average how far away the glucose sensor reading is from a blood
glucose reading The iCan i3 CGM MARD is 8.71%, meaning it may
read 8.71% lower or higher than your blood glucose. For example, if
your blood glucose was 270 mg/dL (15.0 mmol/L), the sensor may read,
on average, 24 mg/dL (1.4 mmol/L) lower or higher.
Performance Metrics* Result Notes
Overall Accuracy 8.71% Mean absolute relative dierence versus across the
range of glucose levels, 36-450 mg/dL(2.0–25.0
mmol/L).
Lower number is better.

 Loading...
Loading...