Precision™ Spinal Cord Stimulator System Clinician Manual
Clinician Manual
97035873-01 68 of 75
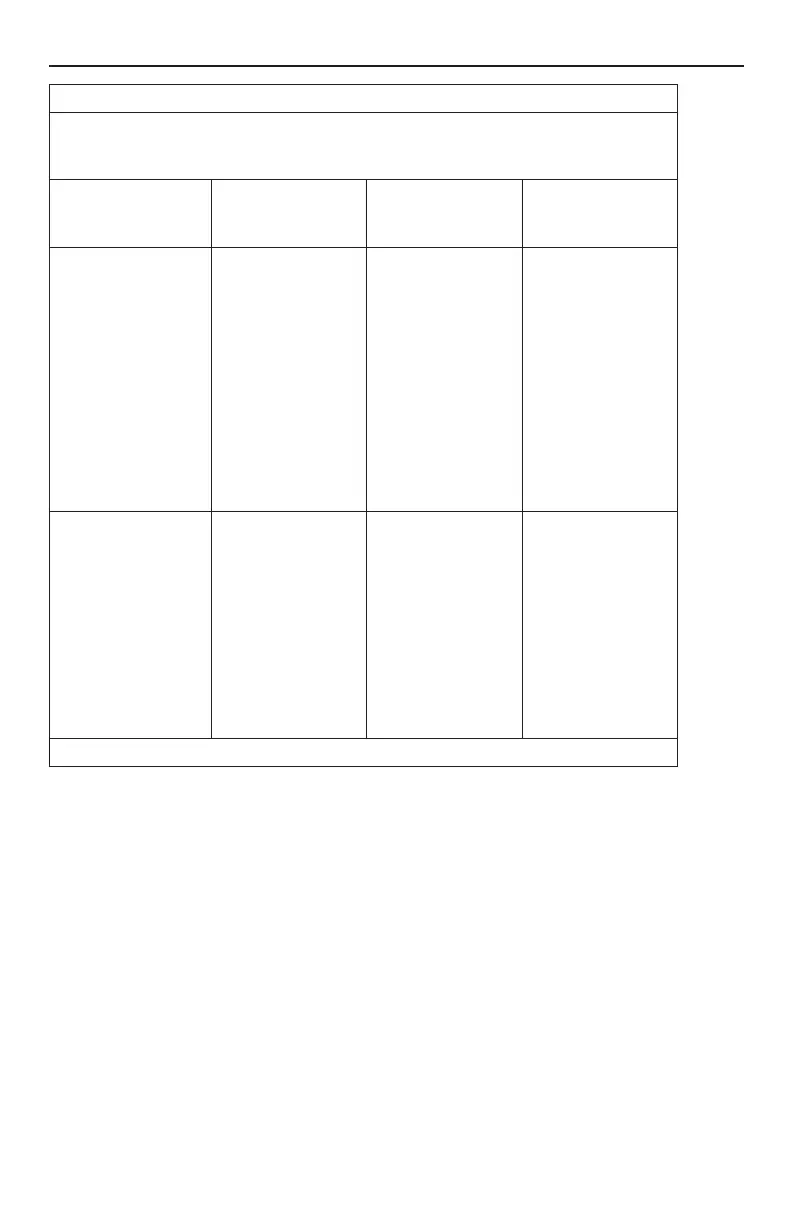
Guidance and manufacturer’s declaration – electromagnetic immunity
The Spectra WaveWriter Spinal Cord Stimulator System is intended for use in the electromagnetic
environment specied below. The customer or the user of the Spectra WaveWriter Spinal Cord
Stimulator System should assure that it is used in such an environment.
Immunity Test IEC 60601 Test Level Compliance Level Electromagnetic
environment
– guidance
Electrostatic discharge
(ESD) IEC 61000-4-2
Air:
± 2 kV, ± 4 kV, ± 8 kV,
± 15 kV
Contact:
± 8 kV
Air:
Remote Control and
Charger: ± 2 kV,
± 4 kV, ± 8 kV, ± 15 kV
ETS: ± 2 kV, ± 4 kV,
± 8 kV
Contact:
Remote Control and
Charger: ± 8 kV
ETS: ± 6 kV
Floors should be wood,
concrete or ceramic tile.
If oors are covered
with synthetic material,
the relative humidity
should be at least
30 %.
Note: Applies to
external devices.
Power frequency
(50/60 Hz)
magnetic eld
IEC 61000-4-8
30 A/m 30 A/m Power frequency
magnetic elds
should be at levels
characteristic of a
typical location in a
typical commercial or
hospital environment.
Magnetic elds from
common appliances are
not expected to affect
the device.
NOTE U
T
is the a.c. mains voltage prior to application of the test level.

 Loading...
Loading...