User Guide • 43
4 Product Information and Safety
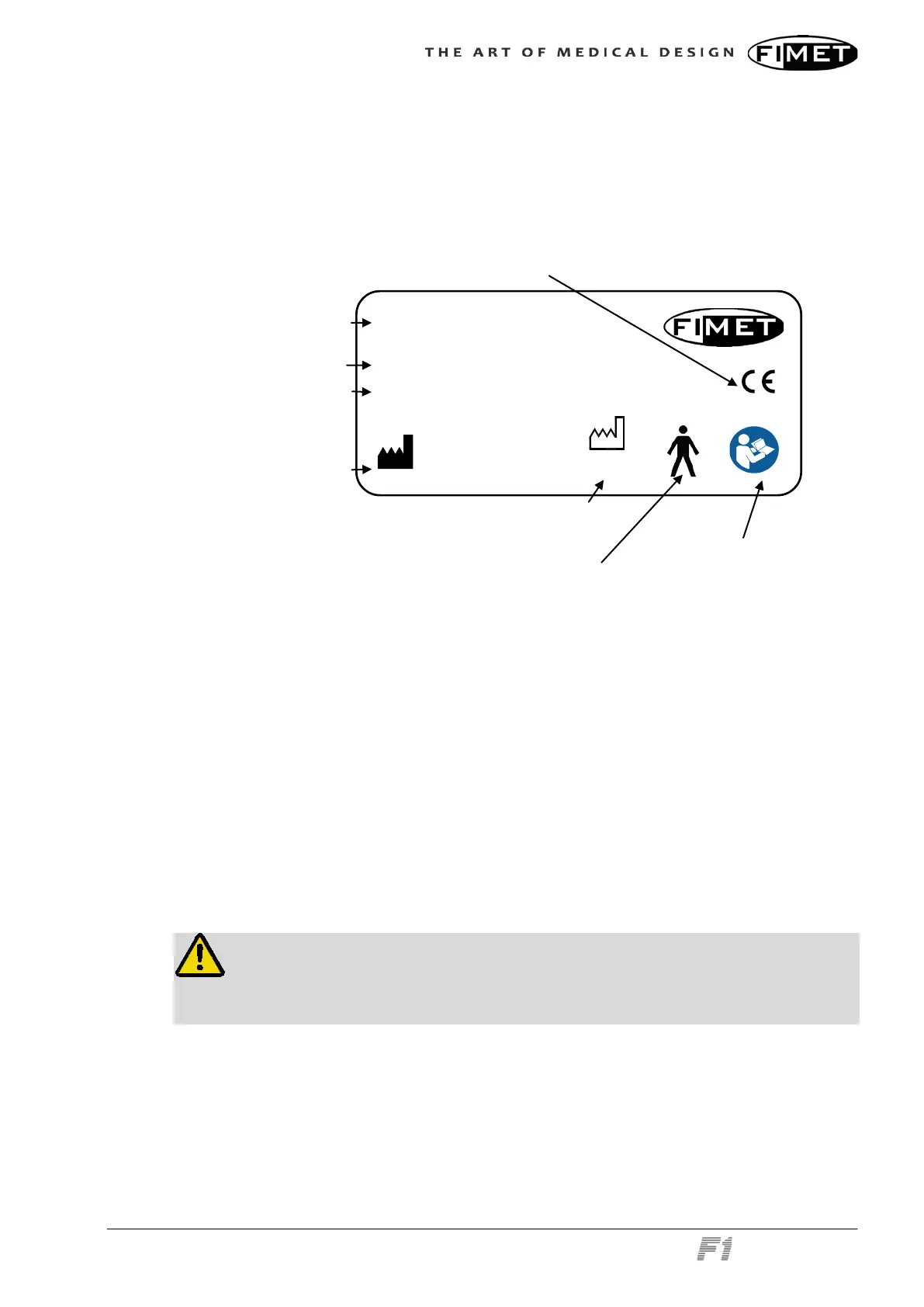
4.1 Device Label
The device label holds the product name, serial number, the year of manufacture, CE-
mark and classifications.
0537
Fimet Oy
Teollisuustie 6
FI-07230 Monninkylä
Finland
2015-01
F1
Dental Treatment System
Serial No: 30212345
Device label
4.2 Intended Use
This dental treatment system is intended for diagnosis, therapy and dental treatment of
persons by properly trained personnel.
4.2.1 Expected Service Life
The expected service life of F1 Dental Treatment system is 10 years. The manufacturer
ensures that the System is safe, for at least this period of time, when serviced according
to the manufacturer’s instructions. In normal conditions, the system is suitable for opera-
tion for a longer period of time than this. The manufacturer ensures that spare parts are
available at least for this time period.
4.2.2 Limitations of Use
Warning!
To avoid the risk of fire, this equipment must not be used with flammable
anaesthetics.
The maximum allowed weight of the patient on the chair with unit attached to it is 135 kg.
The maximum allowed weight on the tray is 1 kg.
The system is designed to be used at altitudes below 2,000 meters. The electrical safety
of the system may be inadequate in altitudes above 2,000 meters.
eration instructions
Protection classification of applied
parts against electric shock
 Loading...
Loading...