GE Analytical Instruments ©2006 13-1 DLM 14291 Rev. A
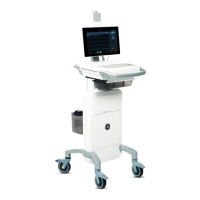
Figure 13- 1: Setup for Nitrate Reduction
13. MEASUREMENT OF NITRATE, NITRITE AND NITRIC OXIDE IN
LIQUID SAMPLES
Nitric oxide reacts with oxyhemoglobin and superoxide anion to form nitrate.
Nitrate is the major oxidation product of NO in some cell culture systems and
in animals and human samples. To measure nitrate, vanadium (III) chloride in
hydrochloric acid is used to convert nitrate to nitric oxide.
2NO
3
-
+ 3V
+3
+ 2H
2
O -> 2NO + 3VO
2
+
+ 4H
+
To achieve high conversion efficiency, the reduction is performed at ~90° C. To
prevent damage to the NOA from the hydrochloric acid vapor, a gas bubbler
filled with aqueous sodium hydroxide is installed between the purge vessel and
the NOA. Vanadium (III)/HCl will also convert nitrite and S-nitrosocompounds to
NO. Nitro-compounds such as nitroarginine, sodium nitropruside, nitroglycerin
are slowly converted to NO by the reagent. This conversion is not quantitative,
but will result in an elevated baseline as the nitro group is slowly converted to
NO. Use of nitroarginine or the methyl ester L-NAME is not recommended when
using VCl
3
/HCl. Methyl arginine
and other NOS inhibitors that
do not contain nitro groups
should be used.
Apparatus for Nitrate
Reduction
Figure 13-1 shows the setup for
nitrate reduction. The outlet
of the purge vessel is
connected to the gas
bubbler/NaOH trap and the
outlet of the bubbler
connected to the inlet of the

 Loading...
Loading...