VF405 Video Frenzel Instructions for Use - English
Date: 2012-08-24 Page 3/33
2. Unpacking and Installation
Unpacking and Inspection
Check box and contents for damage
When the instrument is received please check the shipping box for rough handling and damage. If the box is
damaged it should be kept until the contents of the shipment have been checked mechanically and
electrically. If the instrument is faulty please contact your local distributor. Keep the shipping material for the
carrier’s inspection and insurance claim.
Keep carton for future shipment
The VF405 comes in its own shipping carton, which is specially designed for the VF405. Please keep this
carton. It will be needed if the instrument has to be returned for service.
If service is required please contact your local distributor.
Reporting Imperfections
Inspect before connection
Prior to connecting the product it should once more be inspected for damage. All of the cabinet and the
accessories should be checked visually for scratches and missing parts.
Report immediately any faults
Any missing part or malfunction should be reported immediately to the supplier of the instrument together
with the invoice, serial number, and a detailed report of the problem. In the back of this manual you will find a
"Return Report" where you can describe the problem.
Please use "Return Report"
Please realise that if the service engineer does not know what problem to look for he may not find it, so using
the Return Report will be of great help to us and is your best guarantee that the correction of the problem will
be to your satisfaction.
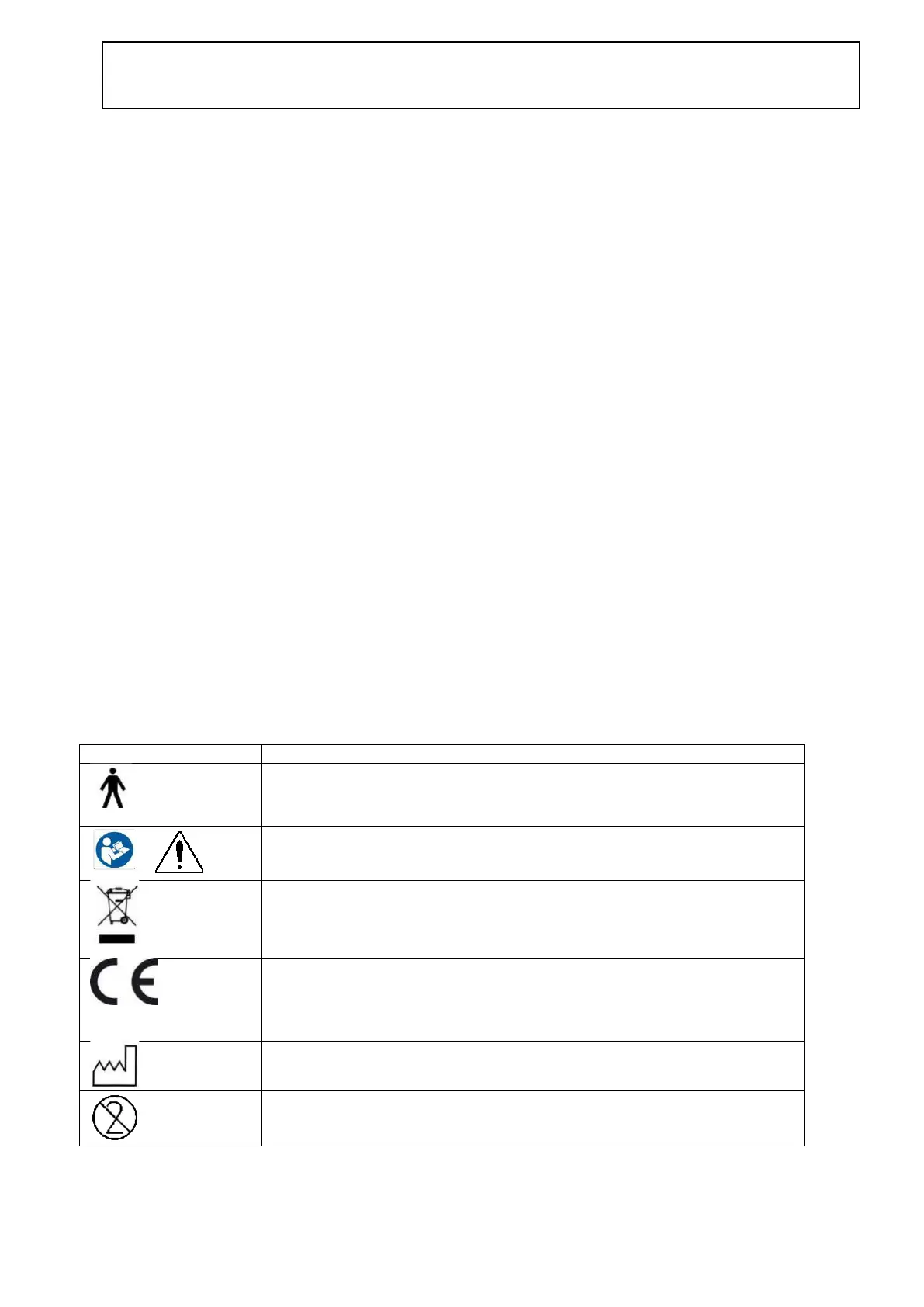
2.1 Marking
The following marking can be found on the instrument:
Type B applied parts.
Patient applied parts that are not conductive and can be immediately
released from the patient.
Refer to instruction manual
WEEE (EU-directive)
This symbol indicates that when the end-user wishes to discard this product,
it must be sent to separate collection facilities for recovery and recycling.
Failing to do so may endanger the environment.
The CE-mark indicates that Interacoustics A/S meets the
requirements of Annex II of the Medical Device Directive
93/42/EEC. TÜV Product Service, Identification No. 0123,
has approved the quality system.
Do not re-use
Parts like ear-tips and similar are for single use only
 Loading...
Loading...











