6. Precautions, Limitations and
Warnings
The qLabs
®
PT-INR monitoring system is intended for in
vitro diagnostics use only. Before using this system to test
PT and INR, take special note of CAUTIONS throughout this
User’s Manual.
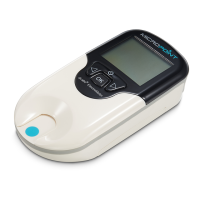
6.1 Care of Your qLabs
®
ElectroMeter
• The qLabs
®
ElectroMeter is a delicate instrument and should be
handled with care. Dropping or other mishandling may cause
malfunction of the qLabs
®
ElectroMeter.
• The qLabs
®
ElectroMeter should be transported in a carrying case
or a secure container.
• DO NOT spill any liquid on the qLabs
®
ElectroMeter. If this should
occur, immediately contact your local distributor from Micropoint
Biotechnologies Co., Ltd.
• DO NOT store the qLabs
®
ElectroMeter below -10 ºC or above
40 ºC.
• DO NOT use the qLabs
®
ElectroMeter for any other types of test
strips not provided by Micropoint Biotechnologies Co., Ltd.
6.2 Patient Health Status
Current patient health status may cause inaccurate or unexpected test
results. It is important to take certain health factors into consideration
when interpreting the test results and deciding on a course of action for
your patients. Failure to do so may lead to an incorrect interpretation of
the PT-INR result.
Self-testing users should discuss their test results with their health care
provider.
6.3 Performing a Test
• The qLabs
®
ElectroMeter should be operated on a level surface that
is free of vibration. Testing on an uneven or unstable surface may
cause inaccurate results. DO NOT hold the qLabs
®
ElectroMeter in
your hands during the testing.
• The blood sample must be applied to the test strip immediately
after collection. Otherwise, the blood sample may begin clotting,
7
EN
 Loading...
Loading...