Fehler! Verwenden Sie die Registerkarte 'Start', um Title;Title dem Text zuzuweisen, der hier angezeigt werden soll. |
12 | 04.10.2021 | Page 43
14. EMC manufacturer's declaration
Electromagnetic emissions and electromagnetic immunity
The RO Medical-Basic device is intended for use in electromagnetic environments as described
below.
The customer or the operator of the RO Medical-Basic should ensure that the device is only
used in such an environment.
This EMC manufacturer's declaration is based on the use of the power supply unit from Phoenix
Contact.
The power supply is installed in the control cabinet.
The cable length between the power supply unit and the cable entry through the housing wall is
150 cm.
Warning
The use of other accessories, other power supply units and cables than specified can lead to
increased emissions and/or reduced interference immunity of the RO Medical-Basic.
Requirements
During the interference immunity tests, the temperature accuracy and conductivity accuracy
were checked.
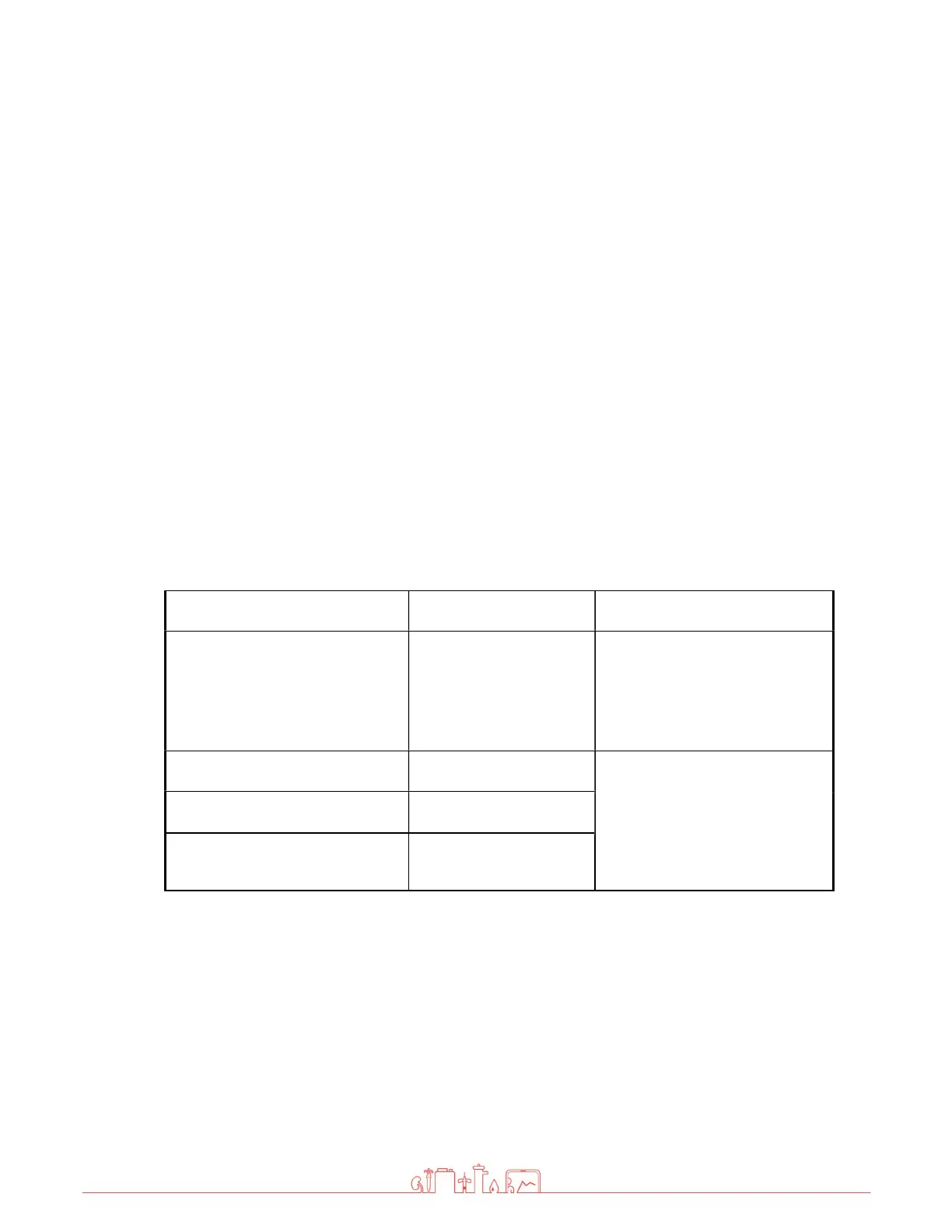
Emission measurement Compliance
Electromagnetic
environment - Guidelines
RF emission in accordance with
CISPR 11 / EN 5511
Group 1 The device only uses RF
energy for its internal
function. Its RF emissions are
therefore very low and
interference to nearby
electronic devices is unlikely.
RF emission in accordance with
CISPR 11 / EN 55011
Class B The device is suitable for use
at any location, including
residential areas and facilities
directly connected to the
public low-voltage grid for
residential buildings.
Harmonics in accordance with
IEC 61000-3-2
Class A
Voltage fluctuations / flickers in
accordance with IEC 61000-3-3
Fulfilled
 Loading...
Loading...