PureWave PEMI User Manual | 06-23 V1.0 Page 3 from 28
PureWave PEMI User Manual
Notes
PureWave PEMI is an AC-powered magnetic field therapy device. The device conforms to the CE Norm and is labeled accordingly with the CE sign.
The manufacturer is only responsible for the safety, operational reliability and functionality of the device if:
• the device is used in accordance with the operating instructions;
• the electrical installation of the location where the device will be used meets the respective current requirements of electrical safety;
• the device is not used in hazardous environments and humid locations;
• mountings, enhancements, re-adjustments, modifications or repair works are carried out only by personnel authorized by the manufacturer;
Technical support may be obtained through the manufacturer, dealers or service organizations authorized by the manufacturer. The product’s duration of life as
scheduled by the manufacturer is 7 years. The PureWave PEMI is an electronic device. Follow your country’s disposal regulations in accordance for electronic
devices.
Comments on electromagnetic compatibility (EMC)
Medical, electrical devices are subject to special precautions concerning the EMC. They must be installed and operated according to the EMC-advice given in the
accompanying documents. In particular medical and electrical devices may be influenced by portable and mobile RF-communication devices.
The manufacturer guarantees the conformity of the unit with the EMC-requirements only when using accessories which are listed in the EC declaration of
conformity. The usage of other accessories my cause an increased emission of electromagnetic disturbances or may lead to a reduced electromagnetic immunity.
The unit must not be arranged physically close to other devices or stacked with other devices. If such an order is necessary, the unit must be observed in order to
check if the device operates as intended. You find more EMC-comments in the chapter “Warnings and Safety Precautions” of this manual as well as in the
Technical Information on the next two pages.
In accordance with the EMC-regulations for medical products we are obliged by law to provide the following information.
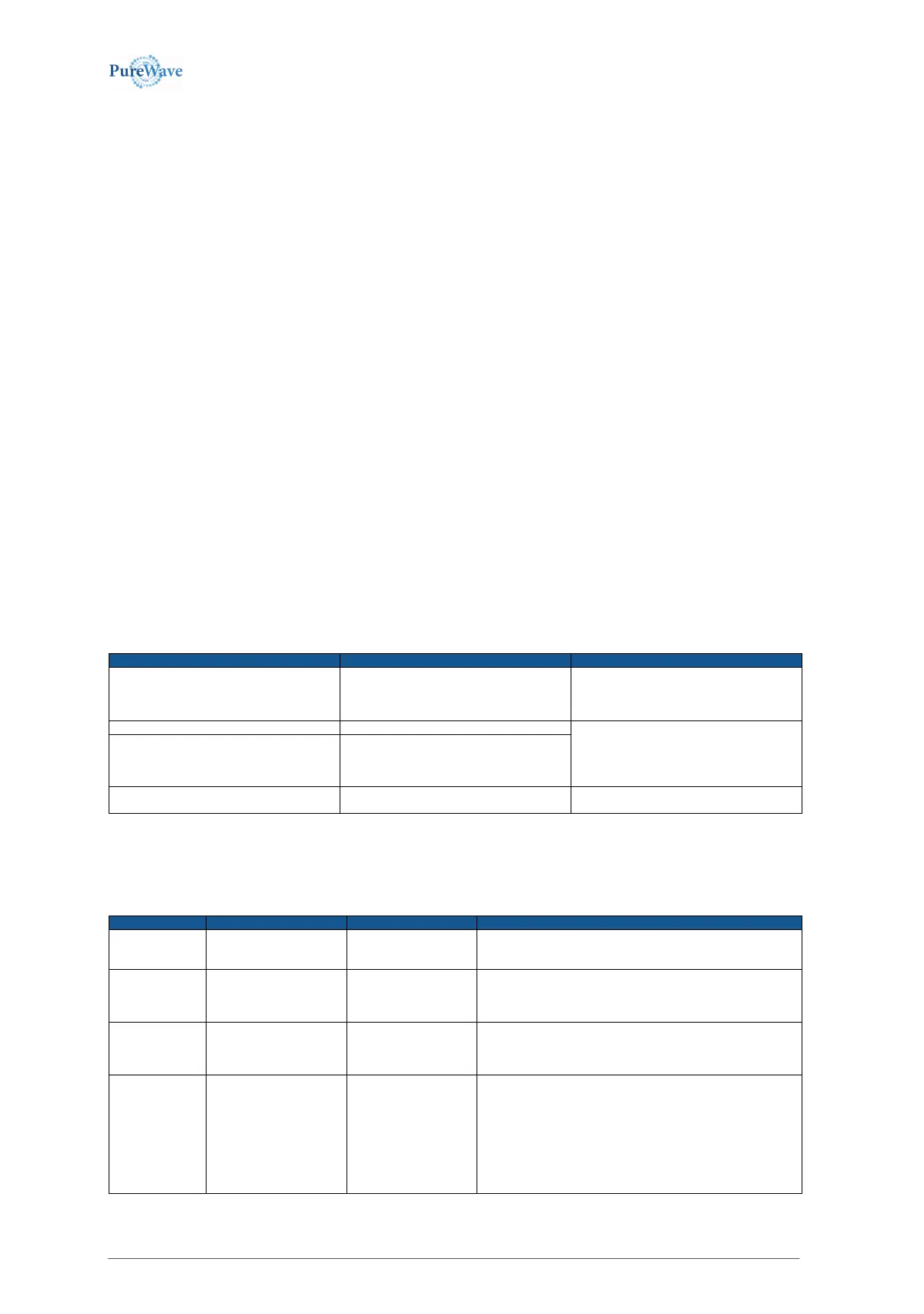
Guidance and manufacturer’s declaration – electromagnetic emissions
The equipment is intended for use in the electromagnetic environment specified below. The customer or the user of the equipment should assure that it is used in
such an environment.
Electromagnetic environment – guidance
The equipment uses RF energy only for its
internal function. Therefore, its RF emissions
are very low and are not likely to cause any
interference in nearby electronic equipment
The equipment is suitable for use in all
establishments, including domestic.
Those directly connected to the
public low-voltage power supply network with
Voltage fluctuation/flicker emissions.
Harmonic emissions,
IEC 61000-3-2 (*)
Voltage fluctuation/flicker emissions,
IEC 61000-3-3 (*)
(*) Note: For devices with a power consumption between 75 W and 1000 W only.
Guidance and manufacturer’s declaration – electromagnetic immunity
The equipment is intended for use in the electromagnetic environment specified below. The customer or the user of the equipment should assure that it is used in
such an environment.
Electromagnetic environment – guidance
Electrostatic
discharge (ESD),
IEC61000-4-2
±8 kV contact discharge
±15 kV air discharge
± 8 kV contact discharge
±15 kV air discharge
Floors should be wood, concrete or ceramic tile. If floors are covered
with synthetic material, the relative humidity should be at least 30%.
Electrical fast
transient/burst,
IEC 61000-4-4
±2 kV for power supply lines
±1 kV for input/output lines
±2 kV for power supply
lines
±1 kV for input/output
lines
Main power source quality should be that of a typical commercial or
hospital environment.
±1 kV outer conductor -
outer conductor
±2 kV outer conductor -
ground
±1 kV outer conductor -
outer conductor
±2 kV outer conductor -
ground
Main power source quality should be that of a typical commercial or
hospital environment.
Voltage dips,
short
interruptions and
voltage variations
on power supply
input lines,
IEC 61000-4-11
0% Ut for ½ cycle in 45°
steps (100% dip)
0% Ut for 1 cycle (100% dip)
70% Ut for 25 or 30 cycle at
50 or 60 Hz (30% dip)
0% Ut for 250 or 300 cycle
at 50 or 60 Hz (100% dip)
0% Ut for ½ cycle in 45°
steps (100% dip)
0% Ut for 1 cycle (100%
dip)
70% Ut for 25 or 30 cycle
at 50 or 60 Hz (30% dip)
0% Ut for 250 or 300
cycle at 50 or 60 Hz
(100% dip)
Main power source quality should be that of a typical commercial or
hospital environment.
If the user of the equipment requires continued operation during
power AC interruptions, it is recommended that the equipment be
powered from an uninterruptible power supply or a battery.
Note: Ut is the a.c. Main power source voltage prior to application of the test level.
 Loading...
Loading...