ABL800 FLEX Reference Manual 2. Amperometric measuring principles
Metabolite electrodes, Continued
Description
(continued)
H
2
O
2
→ 2H
+
+ O
2
+ 2e
−
When a potential is applied to the electrode
chain, the oxidation of H
2
O
2
produces an
electrical current proportional to the
amount of H
2
O
2
, which in turn is directly
related to the amount of glucose or lactate.
To complete the electrical circuit a
reduction reaction (where electrons are
consumed) at the cathode converts Ag
+
(from AgCl) to Ag:
Ag
+
+ e
−
→ Ag
In order to maintain a charge balance between the anode and the cathode, two Ag
+
ions need to be reduced for one molecule of H
2
O
2
to be oxidized.
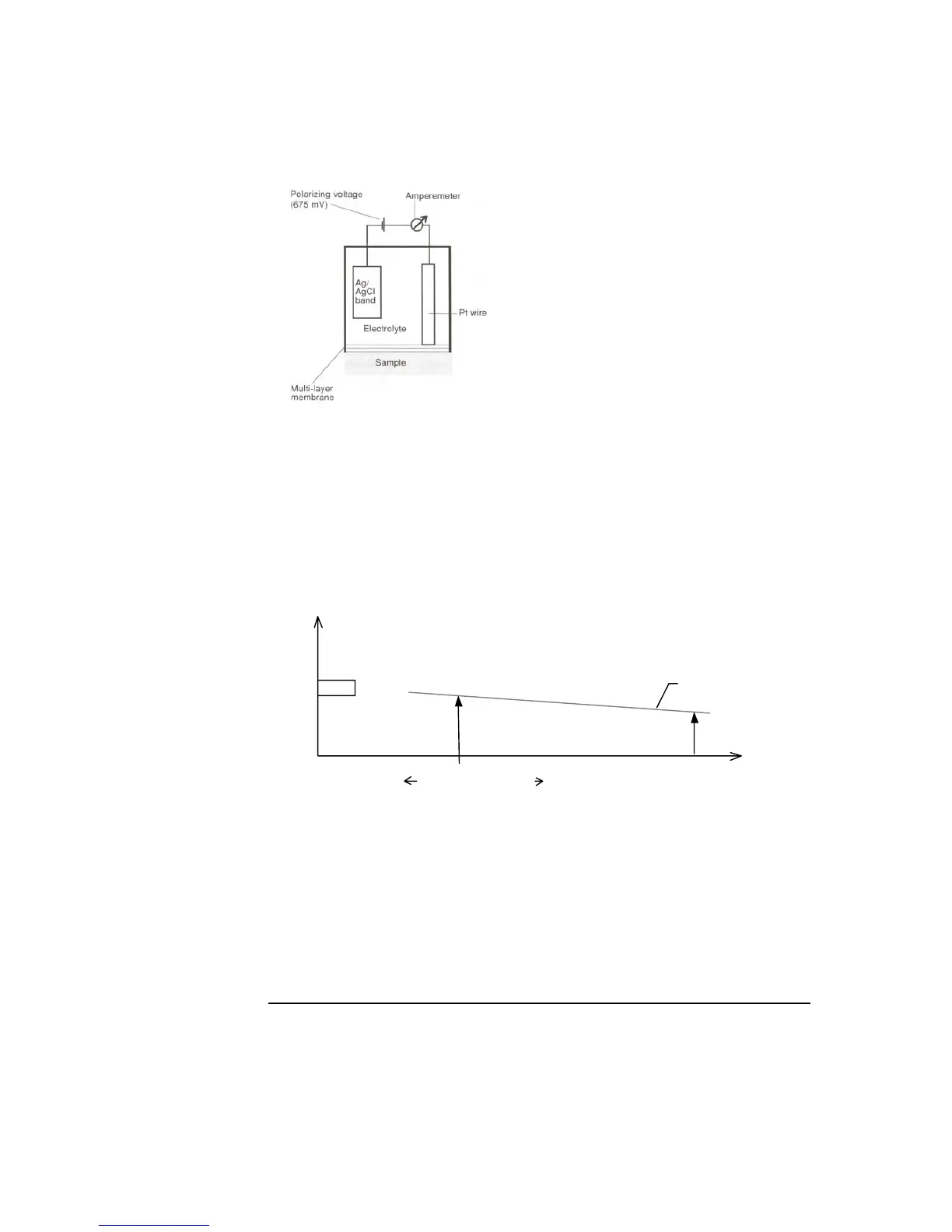
Zero current
The zero current is a small background current measured at the electrode when no
glucose or lactate is present in a solution. As the rinse solution contains no glucose
or lactate, a baseline representing the zero current, I
0
as a function of time (I
0
=
f(t)), is obtained from continuous measurements on the rinse solution.
Rinse
Time
I
(current)
x
xx
x
x
x
x
x
x
x
x
x
x
Extrapolated
base-line
N measurements of I
0
on the rinse solution
t
final
t
mean
I
0
(t)
I
0
(t)
This I
0
baseline is obtained as follows:
• At the end of a rinse, with the rinse solution in the measuring chamber, zero
current of the metabolite electrodes is measured periodically (the intervals
between these measurements become longer if the analyzer is idle).
• The previous N (N = 8) measurements on the rinse solution – before a calibration or
a sample measurement starts - are used to obtain a baseline representing the time
function of I
0
.
Continued on next page
2-13
 Loading...
Loading...