2. Amperometric measuring principles ABL800 FLEX Reference Manual
Metabolite electrodes
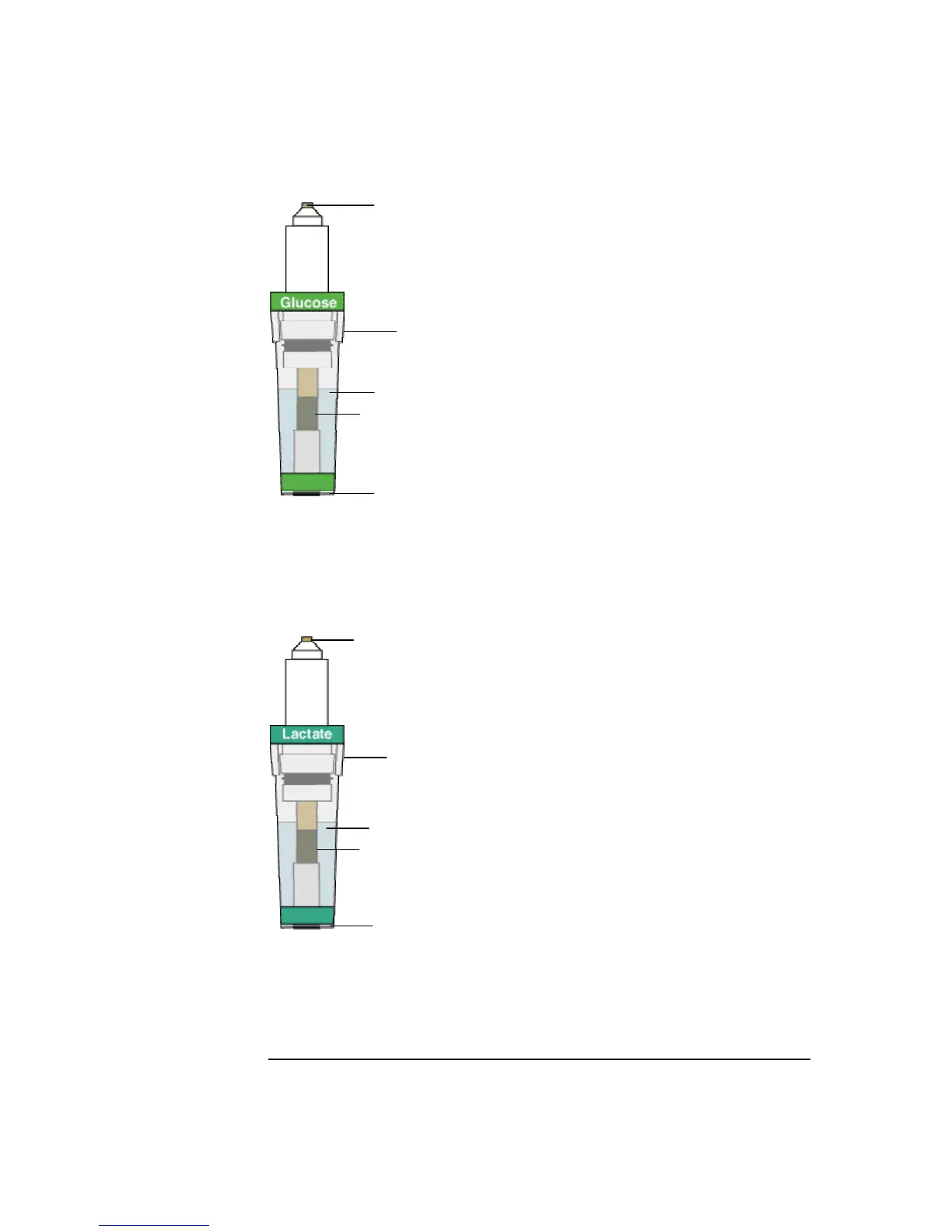
Description
The glucose electrode (E7066) and the
lactate electrode (E7077) have similar
construction described below.
The electrode consists of a silver
cathode and a platinum anode. The
electrode is protected by an electrode
jacket filled with electrolyte solution
and a multi-layer membrane mounted at
the tip.
The membrane consisting of three
layers:
1. outer membrane layer permeable to
glucose/lactate.
2. middle enzyme layer.
3. inner membrane layer permeable to
H
2
O
2
.
A polarization voltage of 675 mV is
applied to the electrode chain and the
current through the chain is measured
by an ampere meter.
Glucose or lactate molecules are
transported across the outer membrane
of the multi-layer membrane.
The enzyme glucose oxidase or lactate
oxidase immobilized between the inner
and outer membrane layers converts the
glucose or lactate according to the
following reactions:
glucose + O
2
→ gluconic acid + H
2
O
2
lactate + O
2
→ pyruvate + H
2
O
2
O
2
for this reaction is supplied by the
outer membrane layer and also by the
oxidation of H
2
O
2
at the Pt anode.
The H
2
O
2
produced by the enzyme
reaction is transported across the inner
membrane to the Pt anode.
 Loading...
Loading...