Page 10
Compliance with the above data should be tested in accordance with
acknowledged analytical methods, by an authorized laboratory.
3.9. Directives and Standards
Every autoclave meets the provisions of the following Directives and is in
compliance with the following Standards:
Tuttnauer. Ltd. company meets the provisions of the following standards:
ISO 13485:2003 (Quality Systems for Medical Devices)
ISO 9001:2008 (Quality Systems)
MDD 93/42/EEC (Medical Device Directive)
Tuttnauer. Ltd. company also works in conjunction with and refers to:
ANSI/AAMI ST55 American Society of Mechanical Engineers
Section VIII, Division 1, for unfired pressure vessels.
EN 13060 Small Steam Sterilizers.
UL UL 61010-1
PED 97/23EEC
IEC IEC 61010-2-040 Safety
ISO 17665-1:2006 (Validation and Routine Control)
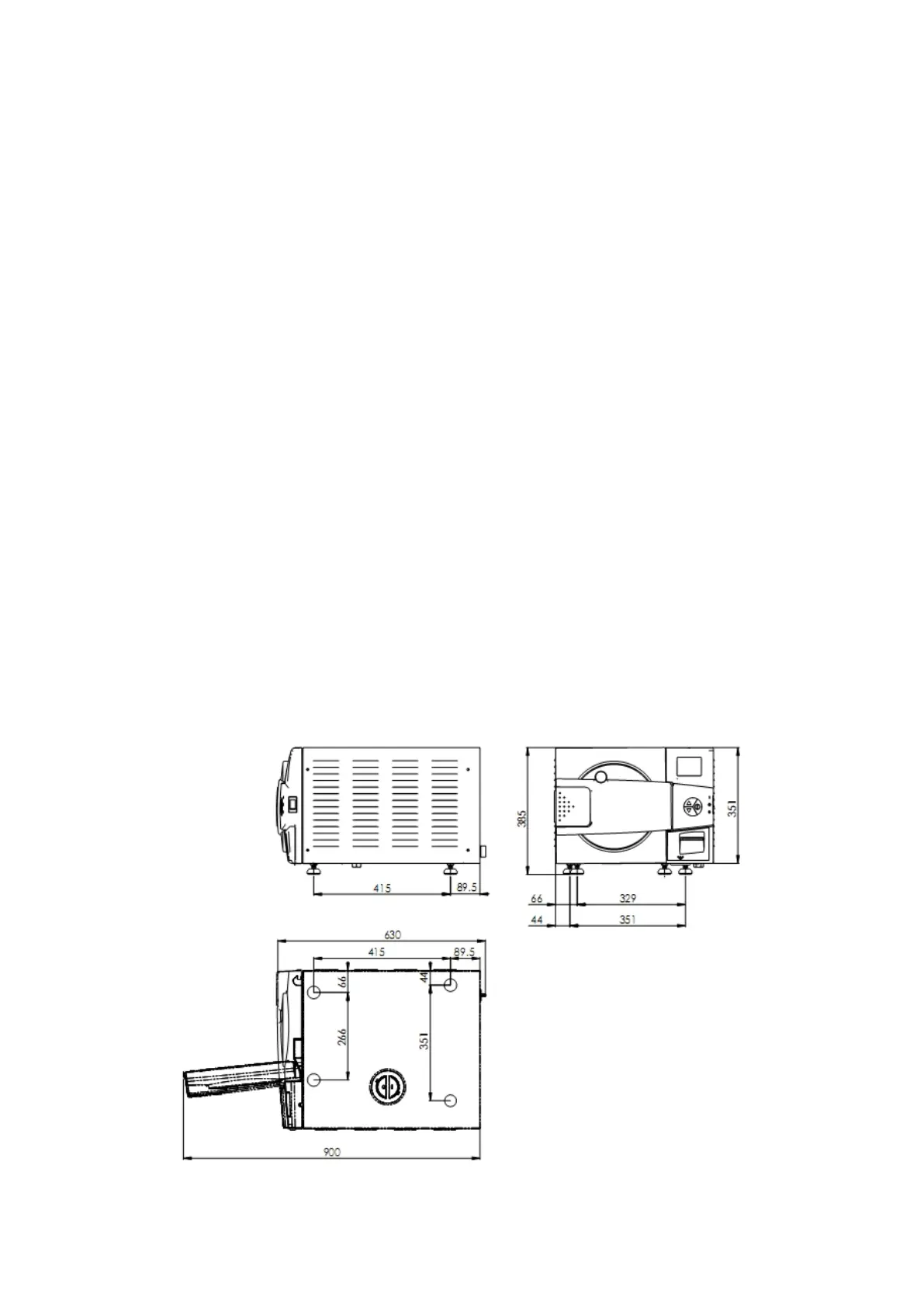
3.10. Overall Dimensions –2340EA-D, EKA-D

 Loading...
Loading...