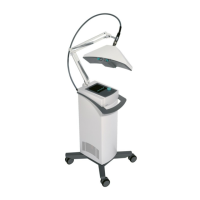
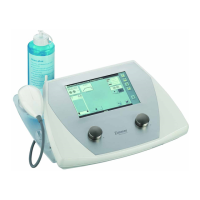
MicroPro 35
© gbo Medizintechnik AG Version 1.4
9.1 Legal requirements and regulations
The device is subject to the provisions of the Medical Device Directive. The safety controls
have to be carried out on the basis of this directive. Thereby, the operator regulation has to
be observed in particular.
9.2 Technical safety checks
Irrespective of the legal rules or beyond the scope of the Medical Device Directive, it is
recommended to have the device checked by the manufacturer or by a maintenance service
authorized by the manufacturer every 12 months.
Refer to separate periodic safety check list for details.
The check includes inter alia the following criteria:
Visual inspection
• Housing undamaged?
• Power cable undamaged?
• Radiator connection sockets undamaged?
• Mains switch OK?
• Radiator cable undamaged?
• Radiator undamaged (no cracks or brittle material)?
Functional test
• Correct function of the indicators
• Operation via touch screen possible
• All fans working
 Loading...
Loading...