6
Introduction
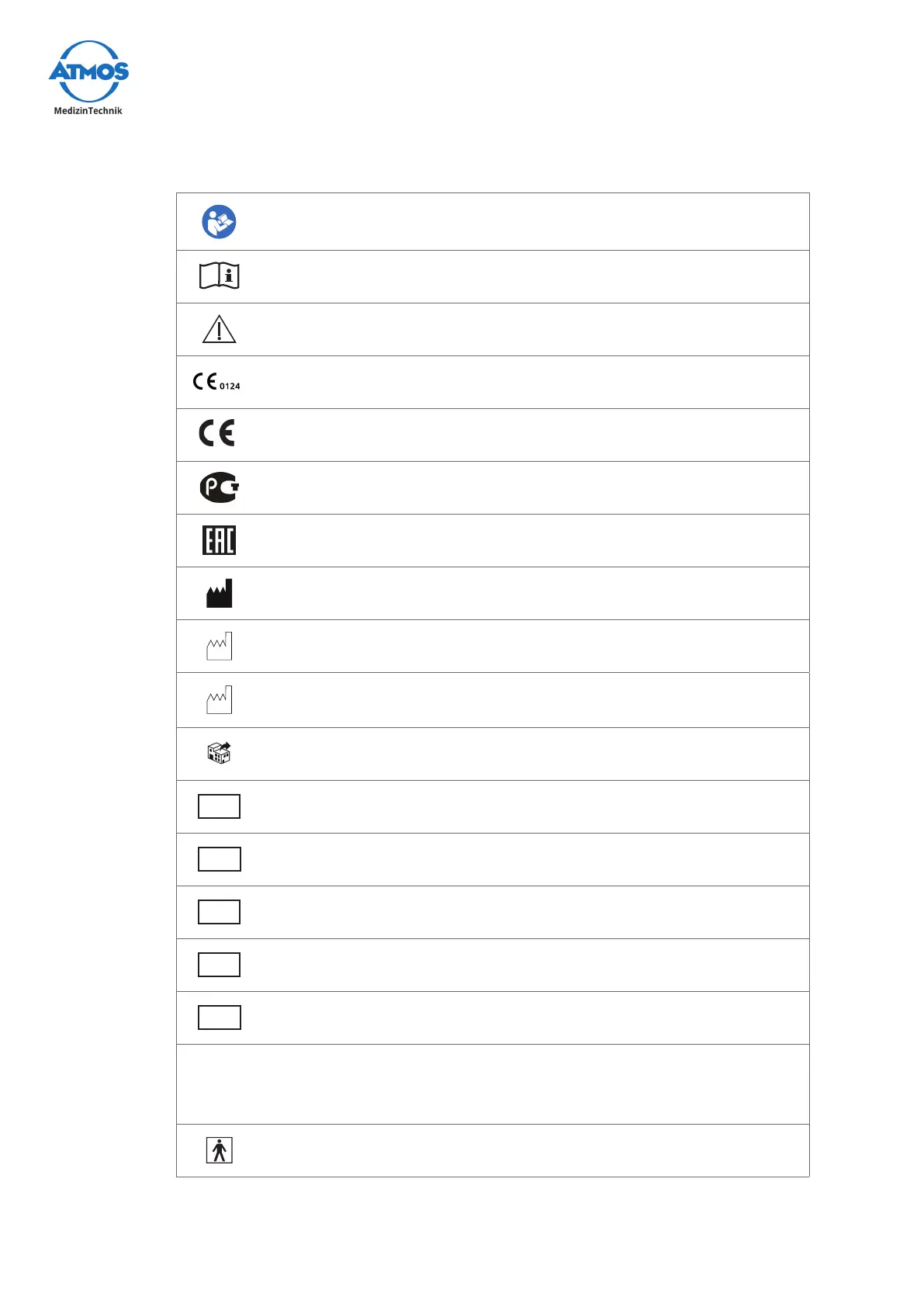
On device, type plate, and packaging
Follow operating instructions (blue)
Observe the operating instructions
Warning; pay special attention
This device complies with the relevant requirements of EU regulations.
This device complies with the relevant requirements of EU regulations.
GOSTCerticate(Russia)
This device complies with the relevant requirements of the Eurasian
Economic Union.
Manufacturer
Date of manufacture
CC
Date of manufacture
Country of manufacture
Distributor
REF
Reference number
UDI
UniqueDeviceIdentierofamedicaldevice
MD
Medical device
SN
Serial number
LOT
Batch code
IP21
Protection against ingress of:
• SolidforeignparticlesØ≥12.5mm
• Dripping water from above
Application part type BF
 Loading...
Loading...