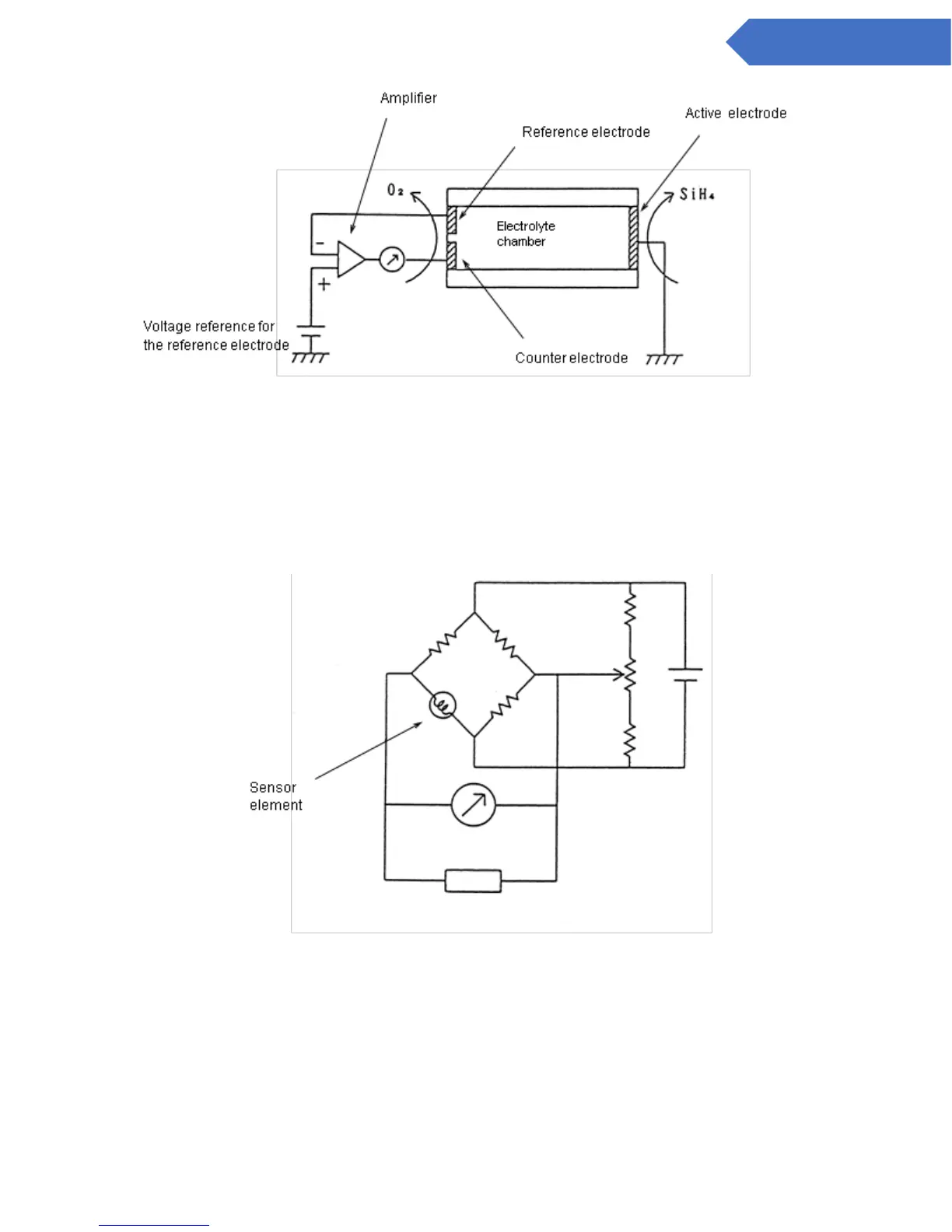
13.2 Hot-wire Semiconductor Sensor
In the hot-wire semiconductor method, a semiconductor sensor is designed to measure the change of
electrical conductivity initiated by adsorption of the electrons of combustible gases onto the surface of a
metal oxide semiconductor heated with a platinum filament. When the semiconductor adsorbs these
electrons, the electron concentration increases and the conductivity of the semiconductor rises. As a result,
the temperature of the semiconductor declines, and the resistance of the platinum filament decreases. This
change is measured as a deviation voltage with a Wheatsone bridge.
Figure 9
13.3 Galvanic Cell Sensor
The galvanic cell sensor consists of noble metal (Pt, Ag) electrode, a base metal (Pb) electrode, and
electrolyte. The noble metal electrode contacts the air through a Teflon membrane.
Since a potential difference is produced between the two electrodes, the following reaction occurs when a
load resistor is connected:
Noble metal electrode O
2
+2H
2
O+4e
−
→4OH
−
 Loading...
Loading...