1-2 Dash™ 3000/4000/5000 2000966-386D
Introduction
Equipment Information
Intended Use
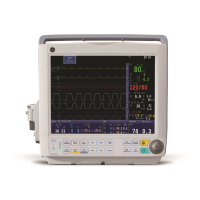
The Dash™ 3000/4000/5000 patient monitor is intended for use under the direct
supervision of a licensed healthcare practitioner. The intended use of the system is to
monitor physiologic parameter data on adult, pediatric and neonatal patients.
The Dash™ 3000/4000/5000 patient monitor is designed as a bedside, portable, and
intra-hospital transport monitor that can operate in all professional medical facilities
including but not limited to: emergency department, operating room, post anesthesia
recovery, critical care, surgical intensive care, respiratory intensive care, coronary
care, medical intensive care, pediatric intensive care, or neonatal intensive care areas
located in hospitals, outpatient clinics, free-standing surgical centers, and other
alternate care facilities.
Physiologic data includes but is not restricted to: electrocardiogram, invasive blood
pressure, noninvasive blood pressure, heart rate, temperature, cardiac output,
respiration, pulse oximetry, carbon dioxide, bi-spectral index, impedance
cardiography, oxygen, and anesthetic agents as summarized in the operator’s manual.
The Dash™ 3000/4000/5000 patient monitor is also intended to provide physiologic
data over the UNITY NETWORK™ indirectly to clinical information systems (via
our Enterprise Gateway) and allow the user to access hospital data at the point-of-
care. The information can be displayed, trended, stored, and printed.
The Dash™ 3000/4000/5000 patient monitor was developed to interface with non-
proprietary third party peripheral devices that support serial data outputs.
Safety Statements
The safety statements presented in this chapter refer to the equipment in general and,
in most cases, apply to all aspects of the monitor. There are additional safety
statements in the parameter chapters which are specific to that monitored parameter.
The order in which safety statements are presented in no way implies order of
importance.
Dangers
Danger statements identify an imminent hazard which, if not avoided, will result in
death or serious injury. No danger statements apply to this monitoring system.
Warnings
Warning statements identify a potential hazard or unsafe practice which, if not
avoided, could result in death or serious injury.
The following warning statements apply to this monitoring system:
 Loading...
Loading...