3
6698G-eIFU-1016
M-LNCS™ Series, LNCS® Series
Adult, Pediatric, Infant, Neonatal and Preterm SpO adhesive sensors
DIRECTIONS FOR USE
Single Patient Use Only
LATEX
Not made with natural rubber latex Non-sterile
INDICATIONS - When Used With Masimo Set® and Masimo compatible Pulse Oximeters:
The M-LNCS™, LNCS® Adult, Pediatric, Infant, Neonatal and Preterm adhesive sensors are indicated for single patient use for continuous noninvasive monitoring of functional oxygen saturation of arterial hemoglo-
bin (SpO) and pulse rate (measured by an SpO sensor) for use with adult, pediatric, infant, and neonatal patients during both no motion and motion conditions, and for patients who are well or poorly perfused in
hospitals, hospital-type facilities, mobile, and home environments.
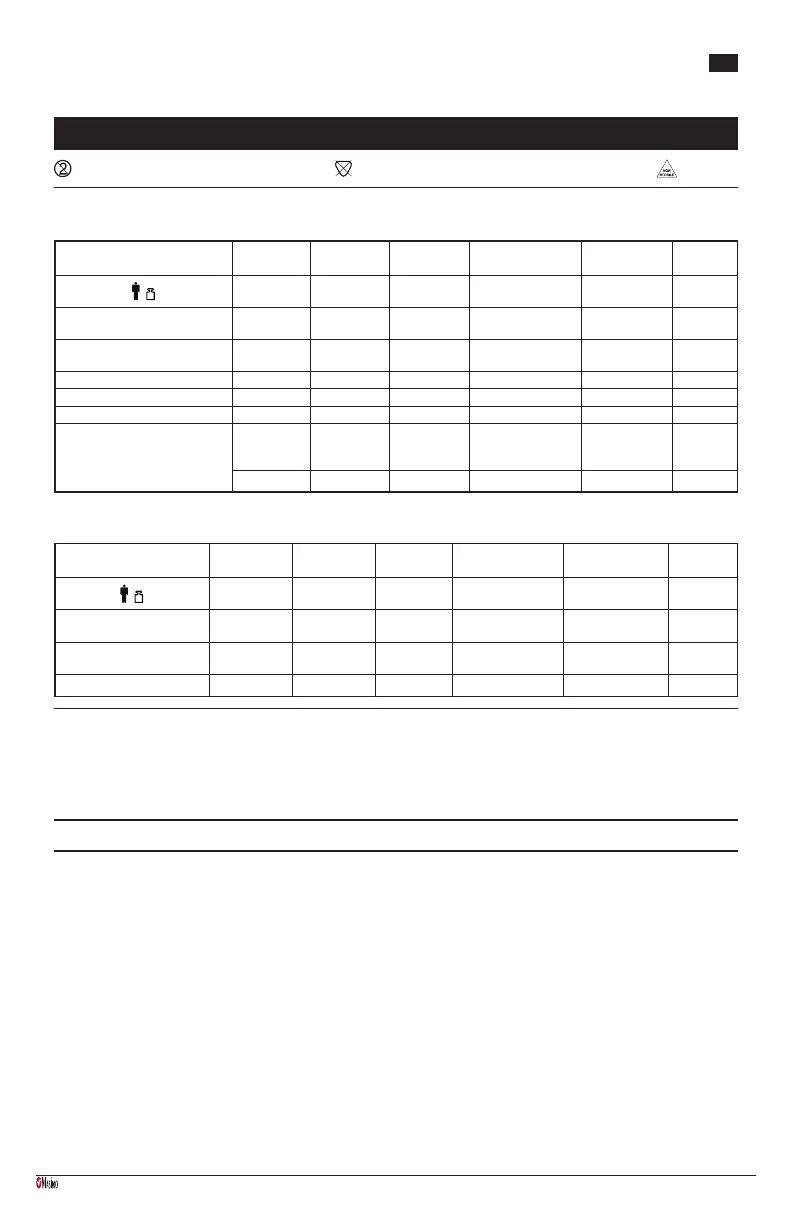
Sensor
Adtx
Adtx-3
Pdtx
Pdtx-3
Inf, Inf-L
Inf-3
Neo, Neo-L
Neo-3
NeoPt, NeoPt-L
NeoPt-3
NeoPt- 500
> 30 kg 10 - 50 kg 3 - 20 kg
< 3 kg
> 40 kg
< 1 kg < 1 kg
Application site Finger or toe Finger or toe
Thumb or
great toe
Neonatal: hand or foot
Adult: nger or toe
Hand or foot Hand or foot
Saturation Accuracy, No Motion ± 2% ± 2% ± 2%
Neonatal ± 3%
Adult ± 2%
± 3% ± 3%
Saturation Accuracy, Motion ± 3% ± 3% ± 3% ± 3% ± 3% ± 3%
Pulse Rate Accuracy, No Motion ± 3 bpm ± 3 bpm ± 3 bpm ± 3 bpm ± 3 bpm ± 3 bpm
Pulse Rate Accuracy, Motion ± 5 bpm ± 5 bpm ± 5 bpm ± 5 bpm ± 5 bpm ± 5 bpm
Low Perfusion Accuracy
SpO2 ± 2% SpO2 ± 2% SpO2 ± 2%
SpO2
Neonatal ± 3%
Adult ± 2%
SpO2 ± 3% SpO2 ± 3%
Pulse ± 3 bpm Pulse ± 3 bpm Pulse ± 3 bpm Pulse ± 3 bpm Pulse ± 3 bpm Pulse ± 3 bpm
INDICATIONS- When used with Nellcor® and Nellcor Compatible Pulse Oximeters:
The M-LNCS, LNCS Adult, Pediatric, Infant, Neonatal and Preterm adhesive sensors are indicated for single patient use for the continuous noninvasive monitoring of functional oxygen saturation of arterial hemoglobin
(SpO) and pulse rate (measured by an SpO sensor) for use with adult, pediatric, infant, and neonatal patients in hospitals, hospital-type facilities, mobile, and home environments.
Sensor
Adtx
Adtx-3
Pdtx
Pdtx-3
Inf, Inf-L
Inf-3
Neo, Neo-L
Neo-3
NeoPt, NeoPt-L
NeoPt-3
NeoPt- 500
> 30 kg 10 - 50 kg 3 - 20 kg
< 3 kg
> 40 kg
< 1 kg < 1 kg
Application site Finger or toe Finger or toe
Thumb or
great toe
Neonatal: hand or foot
Adult: nger or toe
Hand or foot Hand or foot
Saturation Accuracy, No Motion ± 2% ± 2% ± 2%
Neonatal ± 3%
Adult ± 2%
± 3% ± 3%
Pulse Rate Accuracy, No Motion ± 3 bpm ± 3 bpm ± 3 bpm ± 3 bpm ± 3 bpm ± 3 bpm
DESCRIPTION
The M-LNCS, LNCS sensors are for use with instruments containing Masimo SET® oximetry or licensed to use M-LNCS, LNCS sensors and also with Nellcor and Nellcor compatible pulse oximeters, except Nellcor
OxiMax® enabled instruments. Consult individual instrument manufacturer for compatibility of particular instrument and sensor models. Each instrument manufacturer is responsible for determining whether its
instruments are compatible with each sensor model.
The M-LNCS, LNCS series has been validated with Masimo SET Oximetry Technology and on Nellcor’s N-200 Pulse Oximeter. The saturation accuracy of the Neonate and Preterm sensors were validated on adult
volunteers and 1% was added to account for the properties of fetal hemoglobin.
The sensor site must be inspected at least every eight (8) hours; and if the circulatory condition or skin integrity has changed, the sensor should be applied to a dierent site.
WARNING: Masimo sensors and cables are designed for use with instruments containing Masimo SET® oximetry or licensed to use Masimo sensors.
CONTRAINDICATIONS
The M-LNCS, LNCS sensors are contraindicated for patients who exhibit allergic reactions to foam rubber products and/or adhesive tape.
WARNINGS
• All sensors and cables are designed for use with specific monitors. Verify the compatibility of the monitor, cable and sensor before use, otherwise degraded performance and/or patient injury can result.
• The site must be checked frequently or per clinical protocol to ensure adequate adhesion, circulation, skin integrity and correct optical alignment.
• Exercise caution with poorly perfused patients; skin erosion and pressure necrosis can be caused when the sensor is not frequently moved. Assess site as frequently as every (1) hour with poorly perfused
patients and move the sensor if there are signs of tissue ischemia.
• Circulation distal to the sensor site should be checked routinely.
• During low perfusion, the sensor site needs to be assessed frequently for signs of tissue ischemia, which can lead to pressure necrosis.
• With very low perfusion at the monitored site, the reading may read lower than core arterial oxygen saturation.
• Do not use tape to secure the sensor to the site; this can restrict blood flow and cause inaccurate readings. Use of additional tape can cause skin damage, and/or pressure necrosis or damage the sensor.
• Sensors applied too tightly or that become tight due to edema will cause inaccurate readings and can cause pressure necrosis.
• Misapplied sensors or sensors that become partially dislodged may cause incorrect measurements.
• Venous congestion may cause under reading of actual arterial oxygen saturation. Therefore, assure proper venous outflow from monitored site. Sensor should not be below heart level (e.g. sensor on hand
of a patient in a bed with arm dangling to the floor).
• Venous pulsations may cause erroneous low SpO readings (e.g. tricuspid value regurgitation).
• The pulsations from intra-aortic balloon support can be additive to the pulse rate on the oximeter pulse rate display. Verify patient’s pulse rate against the ECG heart rate.
• The sensor should be free of visible defects, discoloration and damage. If the sensor is discolored or damaged, discontinue use. Never use a damaged sensor or one with exposed electrical circuitry.
• Carefully route cable and patient cable to reduce the possibility of patient entanglement or strangulation.
• Avoid placing the sensor on any extremity with an arterial catheter or blood pressure cuff.
• If using pulse oximetry during full body irradiation, keep the sensor out of the radiation field. If sensor is exposed to the radiation, the reading might be inaccurate or the unit might read zero for the duration
of the active radiation period.
• Do not use the sensor during MRI scanning or in a MRI environment.
• High ambient light sources such as surgical lights (especially those with a xenon light source), bilirubin lamps, fluorescent lights, infrared heating lamps, and direct sunlight can interfere with the performance of the sensor.
• To prevent interference from ambient light, ensure that the sensor is properly applied, and cover the sensor site with opaque material, if required. Failure to take this precaution in high ambient light
conditions may result in inaccurate measurements.
• High levels of COHb or MetHb may occur with a seemingly normal SpO. When elevated levels of COHb or MetHb are suspected, laboratory analysis (CO-Oximetry) of a blood sample should be performed.
• Elevated levels of Carboxyhemoglobin (COHb) may lead to inaccurate SpO measurements.
• Elevated levels of Methemoglobin (MetHb) will lead to inaccurate SpO measurements.
en

 Loading...
Loading...