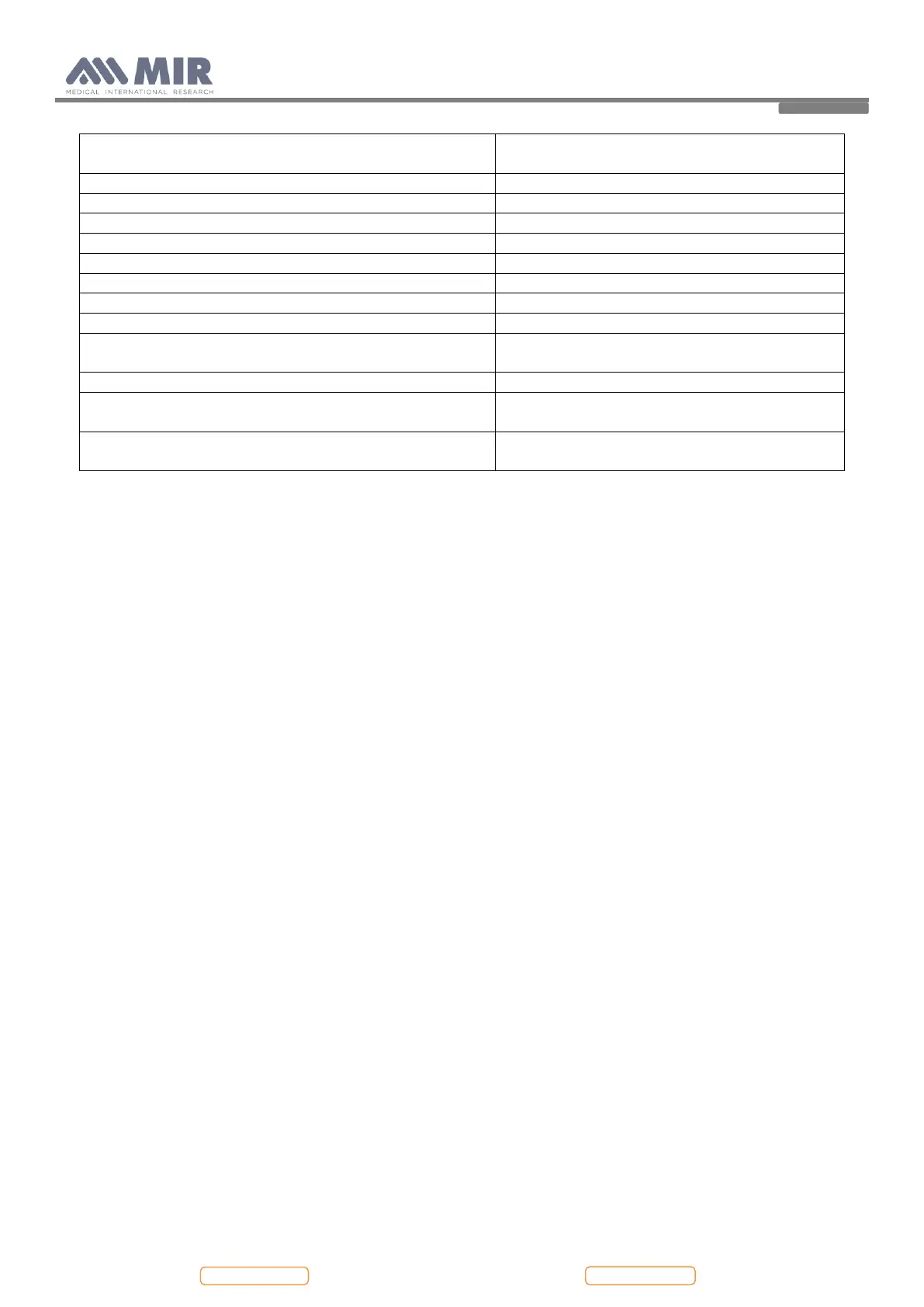
Central unit 99g (including batteries)
Turbine housing 17g
Dynamic resistance at 12 L/s
Type of electrical protection
Level of protection against direct or indirect contact
Level of water ingress protection
IPX1 device, protected against water drops
Level of assurance of use in the presence of an anaesthetic
mix flammable with air or oxygen or protoxide of nitrogen
Apparatus for repeated use
Temperature: MIN -20 °C, MAX +60 °C
Humidity: MIN 10% RH; MAX 95%RH
Temperature: MIN +10 °C, MAX +40 °C
Humidity: MIN 10% RH; MAX 95%RH
1.3.1.2. Battery charger
Battery charger supply: Voltage = 5VDC, Current = 1300 mA
Permissible mains voltage var.: 100 V-240 V
Mains frequency: 50 – 60 Hz
Max. current: 1300 mA at 5 VDC
1.4. STANDARDS APPLIED
According to rule 10 of the European directive 93/42/CEE the device is classified as Class IIa
EN 60601-1 Electronic medical apparatus. Part 1: General safety standards
EN 60601-1-1 Electronic Medical apparatus. Part 1: General safety standards 1. Collateral standard: Safety requirements
for medical electronic systems
EN 60601-1-2 Electronic medical apparatus. Part 1: General safety standards 2. Collateral standard: Electromagnetic
compatibility – Requirements and tests
EN 60601-1-4 Electronic medical apparatus. Part 1: General safety standards 4. Collateral standards: Programmable
medical electronic systems
UNI CEI EN ISO 14971 Medical devices: application of risk management to medical devices
ATS/ERS TASK FORCE: Standardisation of lung function testing (volume 26/numbers 1-5/2005)
Health Canada Medical Device Regulation: P.C. 1998-783 del 7 /5/1998
SANS 451:2008 Spirometry — Generation of acceptable and repeatable spirograms FDA Regulations
Japanese MHLW Ministerial Ordinance 169: 2004
2. HARDWARE DESCRIPTION
Because of the modular design of the spirodoc, the description is on a block diagram level.
BLOCK DIAGRAM
 Loading...
Loading...