1. Potentiometric measuring principles ABL800 FLEX Reference Manual
Electrolyte electrodes, Continued
Stability criteria
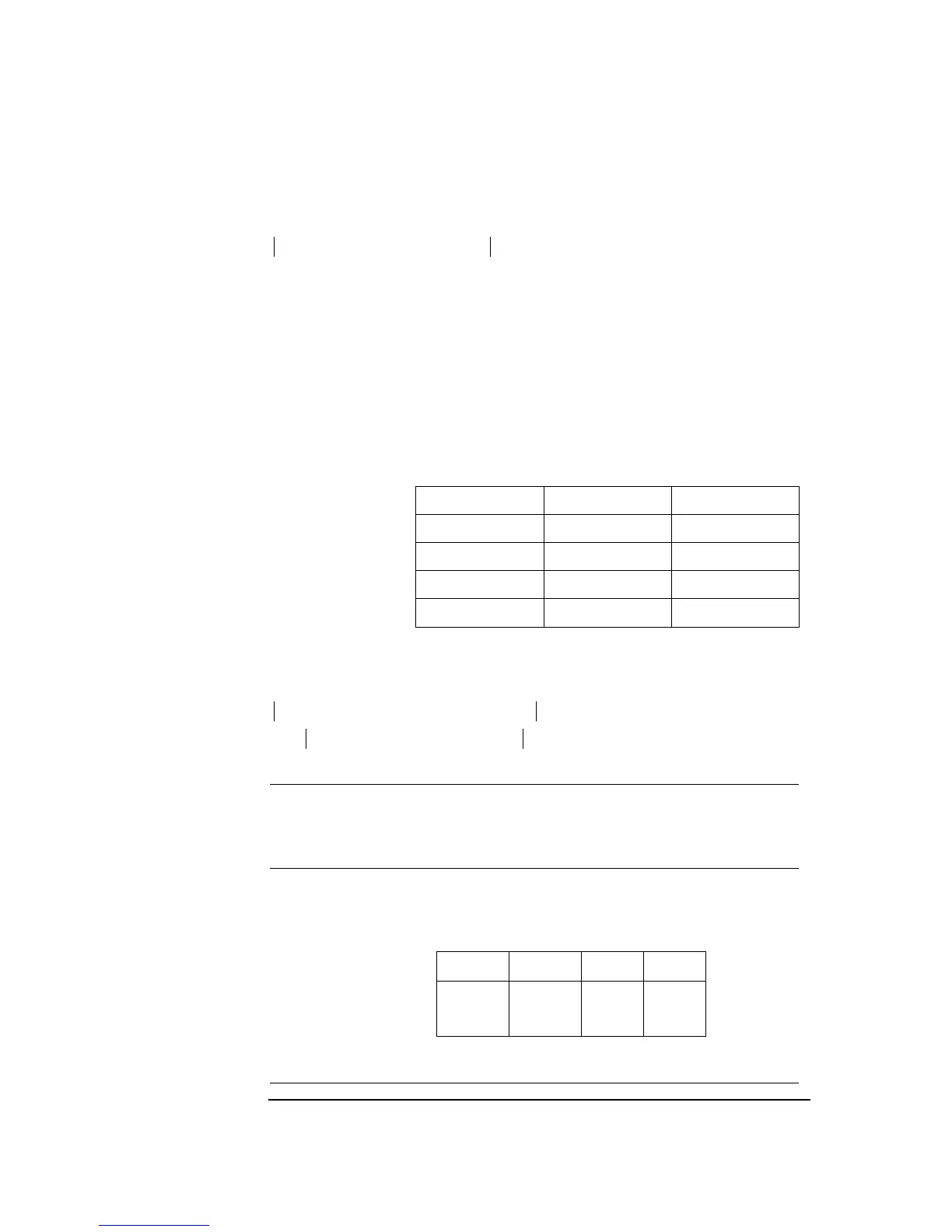
The following stability criterion must be met to obtain a stable electrode response
during calibration:
upd.last)X(Cal,K)upd.iX(Cal,upd.last)X(Cal, ccc ×≤−
This criterion is valid for calibrations using Cal 1 and Cal 2 solutions where:
cX(Cal,upd.last) = Concentration of the electrolyte ion from the last updating
when measuring on calibration solution. (The last
updating is number 30).
cX(Cal,upd.i) = Concentration of the electrolyte ion for a given updating
when measuring on calibration solution. (The relationship
must be fulfilled for at least one of the updating numbers
18 or 19).
K = Constant for the stability criterion.
Electrolyte Ion Cal1 solution Cal2 solution
K
+
0.01 0.01
Na
+
0.01 0.02
Ca
2+
0.02 0.02
Cl
−
0.022 0.022
The following stability criterion must be met to obtain a stable electrode response
during measurement:
()
)()()upd.lastX(sample,K
)upd.iX(sample,upd.last)X(sample,
RinsecXRinsecXc
cc
+−×
≤−
where:
cX(sample,upd.last) = Concentration of the electrolyte ion from the median
of the last 5 updatings (for Ca
2+
: 3 last updatings)
when measuring on a sample. The last updating
number is 30 (or 10 for some micromodes).
cX(sample,upd.i) = Concentration of the electrolyte ion for a given
updating when measuring on a sample. (The
relationship must be fulfilled for at least one of the
updating numbers shown below).
K
+
Na
+
Ca
2+
Cl
−
22 22 26 22
23 23 27 23
In some micromodes, substract 20 from number
above.
Continued on next page
1-32
 Loading...
Loading...