Multi 197i Operation
21
ba75339e04 07/2009
4.3.4 pH calibration
Why calibrate? pH electrodes age. This changes the asymmetry (zero point) and slope
of the pH electrode. As a result, an inexact measured value is dis-
played. Calibration determines the current values of the asymmetry
and slope of the electrode and stores them in the measuring instru-
ment. Thus, you should calibrate at regular intervals.
When to calibrate? z After connecting another electrode
z When the sensor symbol flashes, i.e. after the calibration interval
has expired
Calibration points Calibration can be made with one or two buffer solutions (single-point
or two-point calibration). The measuring instrument determines the fol-
lowing values and calculates the calibration lines as follows:
AutoCal TEC is specially matched to the WTW technical buffer solutions as a fully au-
tomatic two-point calibration. The buffer solutions are automatically
recognized by the measuring instrument. Depending on the instrument
setting (see section 4.9 C
ONFIGURATION), the instrument displays the
relevant buffer nominal value or the current electrode voltage in mV.
The calibration can be terminated after the first buffer solution. This cor-
responds to a single-point calibration. To do this, the instrument uses
the Nernst slope (-59.2 mV/pH at 25 °C) and determines the asymme-
try of the electrode.
AutoRead The calibration procedure automatically activates the AutoRead func-
tion.
The current AutoRead measurement can be terminated at any time
(accepting the current value) by pressing <RUN/ENTER>.
Displaying the calibra-
tion data
You can view the data of the last calibration on the display. The pro-
ceeding is described on page 50.
Printing the
calibration protocol
The calibration protocol contains the calibration data of the current cal-
ibration. You can transmit the calibration protocol to a printer via the se-
rial interface (see O
UTPUTTING THE CALIBRATION PROTOCOL ON THE
INTERFACE, page 53).
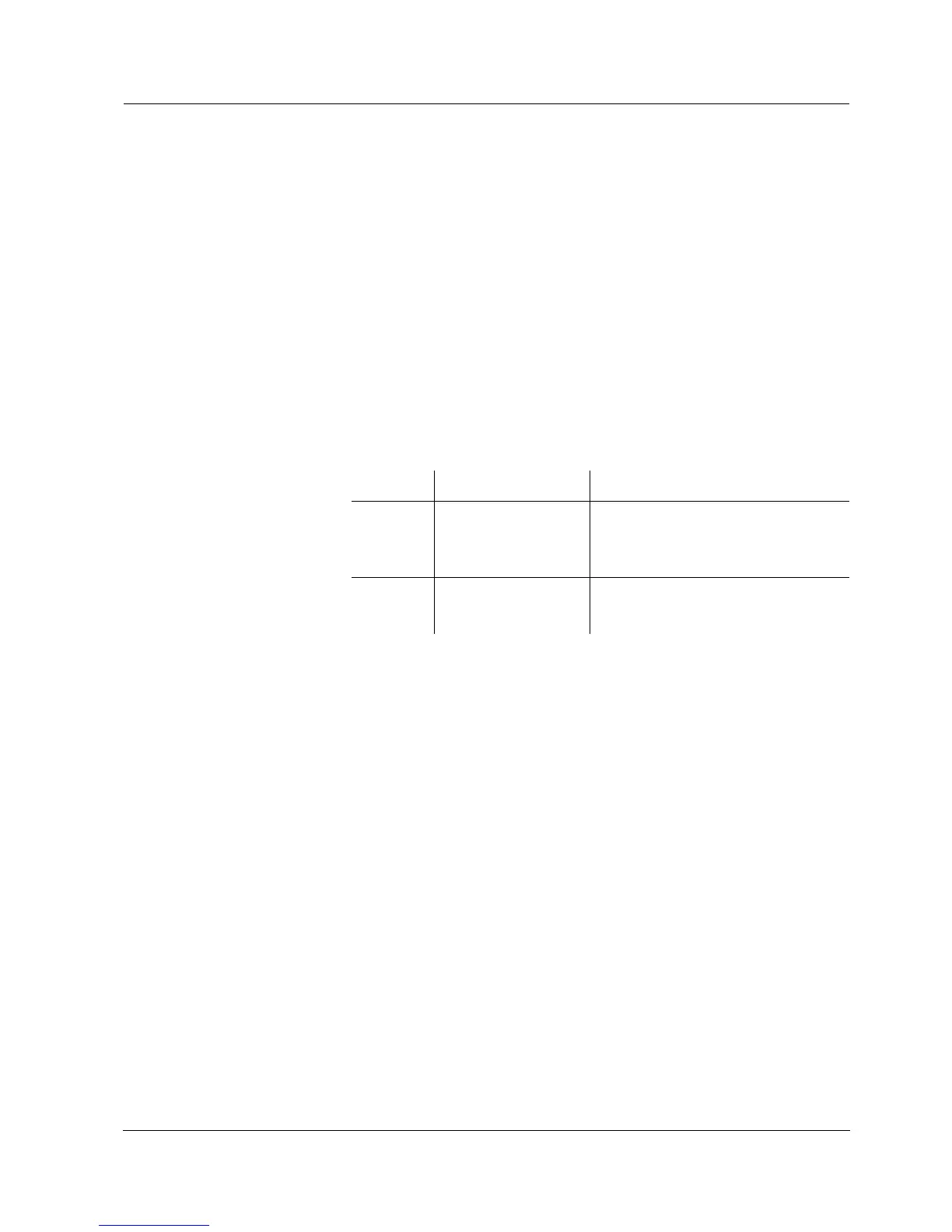
Determined values Values of the calibration lines
1-point ASY z Asymmetry = ASY
z Slope = Nernst slope (59.16 mV/
pH at 25 °C)
2-point ASY
SLO
z Asymmetry = ASY
z Slope = SLO
 Loading...
Loading...