11.17 ELECTROMAGNETIC COMPATIBILITY
All the information below is based on the requirements of standards to which the manufacturers of electrical medical devices
must adhere (as stated in standard IEC60601-1-2).
The medical device complies with the electromagnetic compatibility standards in force. However, the user will make sure that
any electromagnetic interference does not create an additional risk, such as radiofrequency transmitters, or other electronic
devices.
This chapter contains the information required for you to install and use your medical device in optimum conditions in terms
of electromagnetic compatibility.
Some types of mobile telecommunication devices, such as mobile phones, may interfere with the medical device. The
separation distances recommended in this chapter MUST be respected.
The medical device must not be used near another device or placed on top of it. If this cannot be avoided, correct operation
of the device in operating conditions must be checked prior to use.
The use of accessories other than those specied or sold by ACTEON® Imaging as replacement parts, may increase the
transmission or reduce the immunity of the medical device.
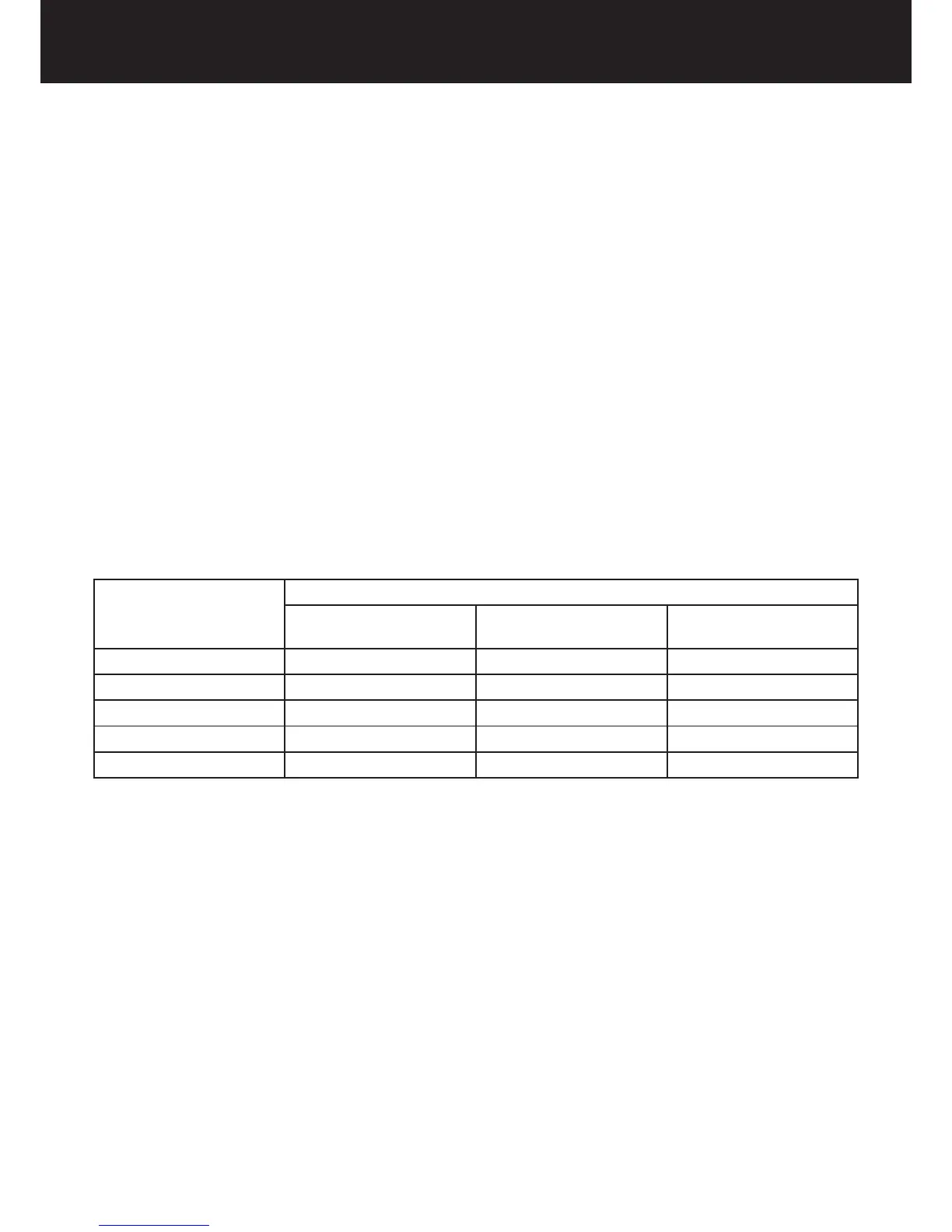
11.17.1 RECOMMENDED SEPARATION DISTANCES
The medical device is designed to be used in an electromagnetic environment in which interferences caused by RF radiation
are controlled.
The user or installer of the medical device may help to prevent electromagnetic interference by maintaining a minimum
distance, depending on the maximum power of the handheld and mobile radiofrequency transmission equipment
(transmitters), between the medical device and the equipment as recommended in the table below.
Rated maximum output
power of the transmitter
[W]
Separation distance according to transmitter frequency [m]
150 kHz - 80 MHz
d = 1,2 √P
80 MHz - 800 MHz
d = 1,2 √P
800 MHz - 2.5 GHz
d = 2,3 √P
0.01 0.12 0.12 0.24
0.1 0.38 0.38 0.73
1 1,2 1.2 2.3
10 3,8 3.8 7.3
100 12 12 23
In the event of transmitters whose maximum nominal output power coecient does not fall within the indicated parameters,
the recommended separation distance in metres (m) can be determined by means of the equation corresponding to the
frequency of the transmitter, where (P) is the maximum output power coecient of the transmitter in watts (W) according to
the information provided by the manufacturer.
Note 1: At 80 MHz and 800 MHz apply the separation distance corresponding to the highest frequency range.
Note 2: These guidelines may not apply in every situation. Electromagnetic propagation is affected by absorption and reection from
structures, objects and people.
The electromagnetic propagation is affected by absorption and reection from structures, objects and people.
11.17.2 ELECTROMAGNETIC EMISSIONS
The medical device is designed for use in the electromagnetic environment described in the table below. The user and/or
installer must ensure that the medical device is used in the environment described below.
 Loading...
Loading...