Appendix B Example RQ Experiment
Applied Biosystems 7300/7500/7500 Fast Real-Time PCR System Relative Quantification Getting Started Guide 83
Notes
B
b. Prepare the cDNA archive plate by pipetting
into each well of the plate:
•50 µL RT master mix
•30 µL nuclease-free water
•20 µL RNA sample
Make sure the total amount of RNA is 10 to
100 ng for each 100-µL reaction.
c. Program the thermal cycler using the
indicated parameter values for the RT step
of the two-step RT-PCR method.
Note: You have the option to use one-step
RT-PCR, as explained in “Selecting One- or
Two-Step RT-PCR” on page 12.
d. Store the cDNA at −20 ° C until use.
4. Prepare the PCR master mix as indicated in the
table to the right.
See Chapter 4 on page 22 for more information.
Note: The concentrations of TaqMan
®
Gene
Expression Assays and TaqMan
®
Custom Gene
Expression Assays are specified in the product
insert. The concentrations of primers and probes
designed with Primer Express software follow
the universal assay conditions described in
Chapter 4.
CHEMICAL HAZARD.
TaqM an
®
Universal PCR Master Mix (2✕) No
AmpErase
®
UNG may cause eye and skin
irritation. Exposure may cause discomfort if
swallowed or inhaled. Read the MSDS, and
follow the handling instructions. Wear
appropriate protective eyewear, clothing, and
gloves.
Liver Kidney Bladder
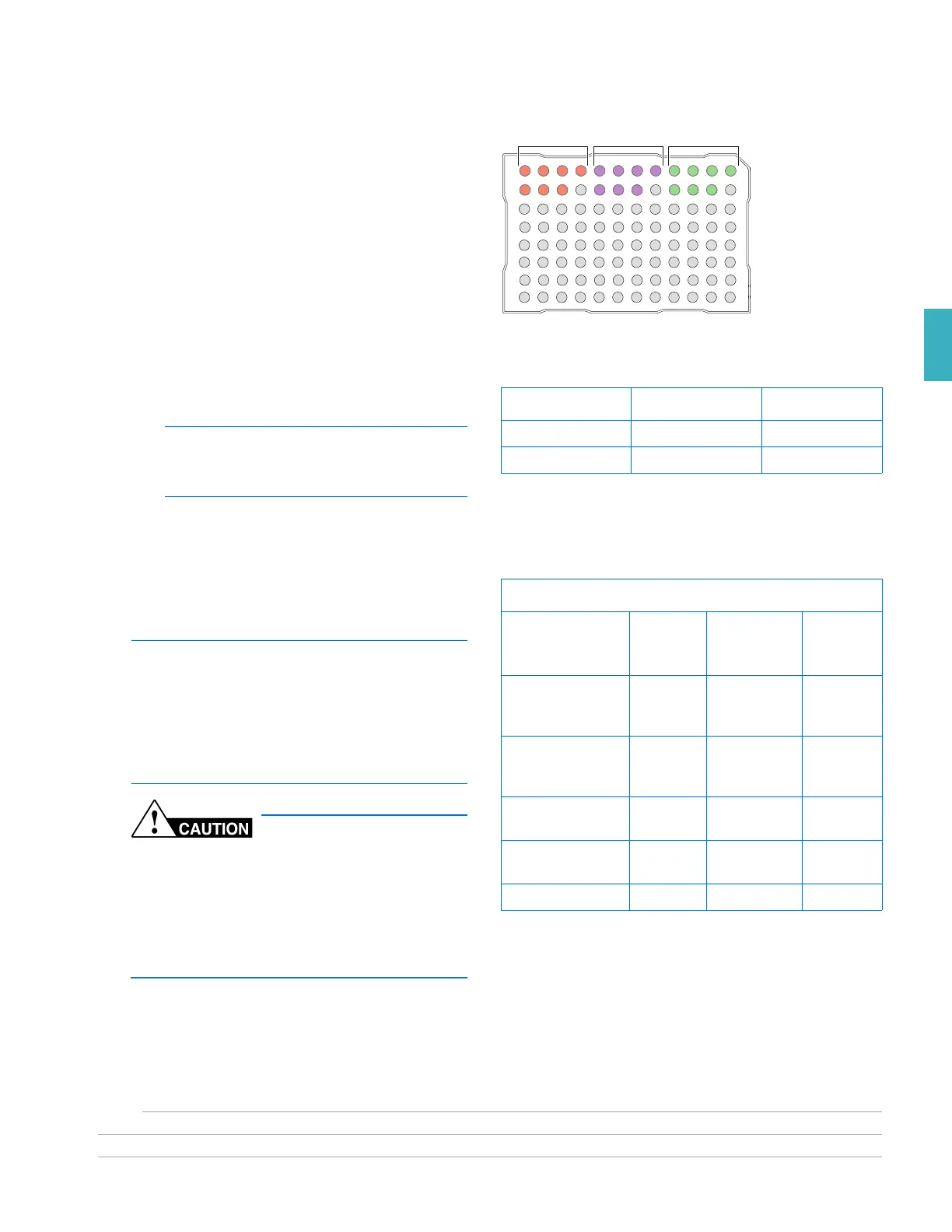
GR2322
Step Type Time Temperature
HOLD 10 min 25 ° C
HOLD 120 min 37 ° C
PCR Master Mix - Standard
Reaction
Component
µL/
Sample
µL/ 5
Reactions
§
§ 24 master mixes are prepared, one for each of 23 genes plus the
endogenous control. Volume for five reactions (4 replicates plus
extra) to account for pipetting losses.
Final
Concen-
tration
Taq Ma n
Universal PCR
Master Mix (2✕)
25.0 125.0 1✕
20✕ TaqM a n
®
Gene Expression
Assay Mix
‡
‡ Contains forward and reverse primers and labeled probe.
2.5 12.5 1✕
cDNA sample 5.0 25.0 10 to
100 ng
Nuclease-free
water
17.5 87.5 —
Total 50.0 250 —

 Loading...
Loading...