User Manual D2 PHASER
DOC-M88-EXX141 V5 – 01.2015 28
Characteristic Spectrum
High-energy electrons can also interact with the atoms of the metal target to produce another type of
radiation called “characteristic” radiation because the wavelengths of the photons emitted are
characteristic of the anode metal.
In this case, the incident electron collides with an electron of the first, innermost electron shell of an
atom and knocks it out of its orbit. To restore stability to the energy state of the atom, the ejected
electron is replaced by an electron from an outer shell. Because the energy levels of shells increases
with their distance from the nucleus of the atom, the transition of a replacement electron from an outer
to an inner shell is accompanied by a loss in energy, which is released in the form of X-rays.
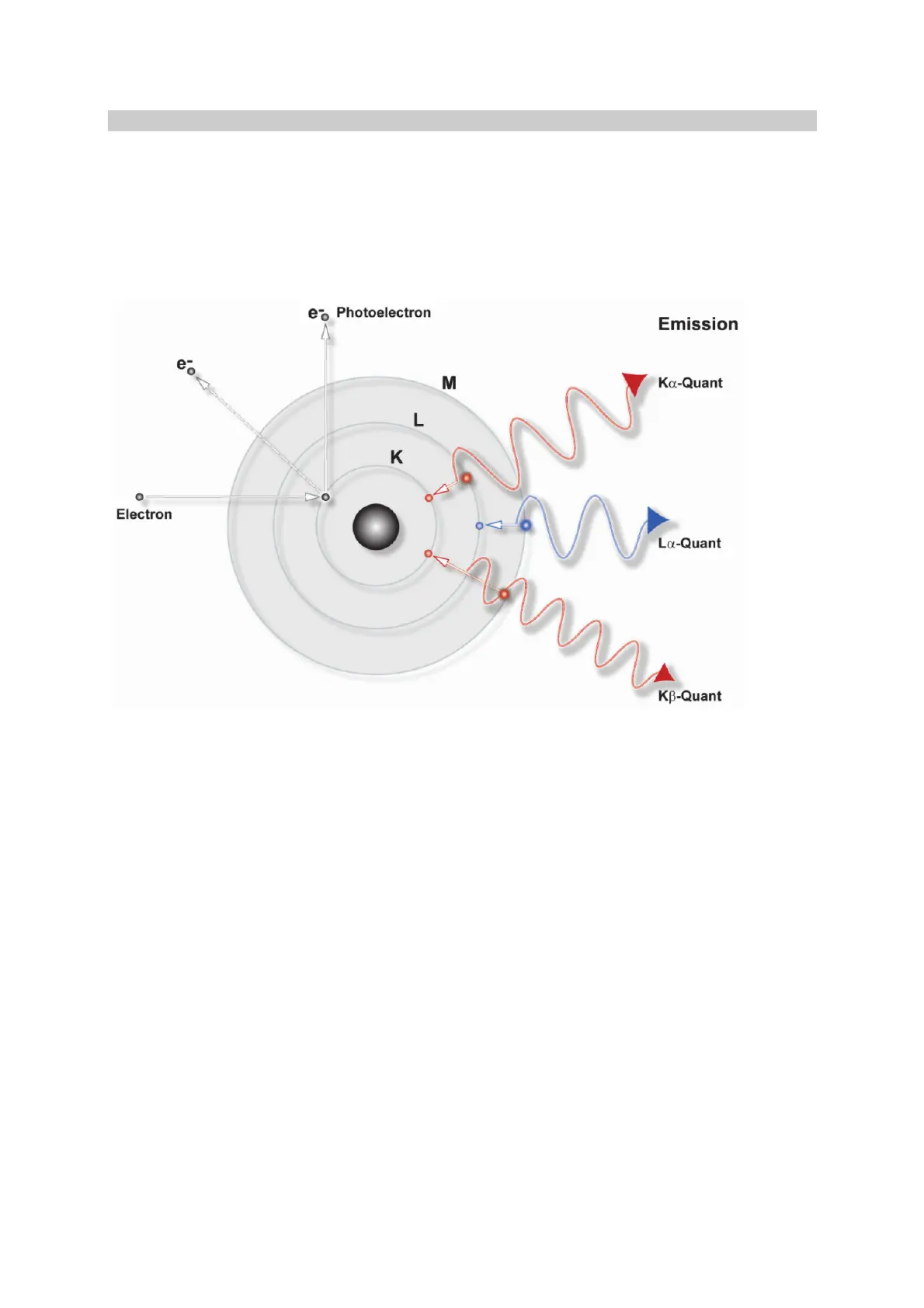
Fig. c: Production of characteristic radiation - simplified representation.
Fig. c is a simplified representation of the process by which characteristic radiation is produced
through the transition of electrons at the atomic level. In this diagram the conventional notation for the
electron shells and the photons produced by the transition of their respective electrons has been
adopted. Kα radiation is produced by an electron transfer from shell L to shell K, Kβ by a transfer from
shell M to shell K, and Lα by a transfer from M to L. Note that the photons have different characteristic
wavelengths.
In fact, on closer examination of the atom and its electron shells, it can be seen that the shells are
divided into subshells as shown in Fig. d below. For this reason, Kα radiation can be divided into Kα
1
and Kα
2
radiation, Kβ radiation into Kβ
1
and Kβ
2
radiation and so on. The difference between the
wavelengths of photons produced by the transfer of electrons of different subshells within the same
shell is very small.
 Loading...
Loading...










