Dakewe (Shenzhen) Medical Equipment Co., Ltd
13.Electromagnetic Compatibility Indicators
Note:
[HP300 Plus] [Automatic Tissue Processor] shall meet the emission and immunity requirements
specified in IEC61326-2-6, as shown in the table below.
The user shall be responsible for ensuring the electromagnetic compatibility of the environment
for the equipment to work normally.
It is recommended to evaluate the electromagnetic environment before the equipment is used.
Warning:
[HP300 Plus] [Automatic Tissue Processor] is designed and and tested according to Class A
equipment in CISPR 11. In the home environment, the equipment may cause radio interference, so
that protective measures should be taken.
It is prohibited to use the equipment next to an intense radiation source (such as an unshielded RF
source), otherwise the normal operation of the equipment will be affected.
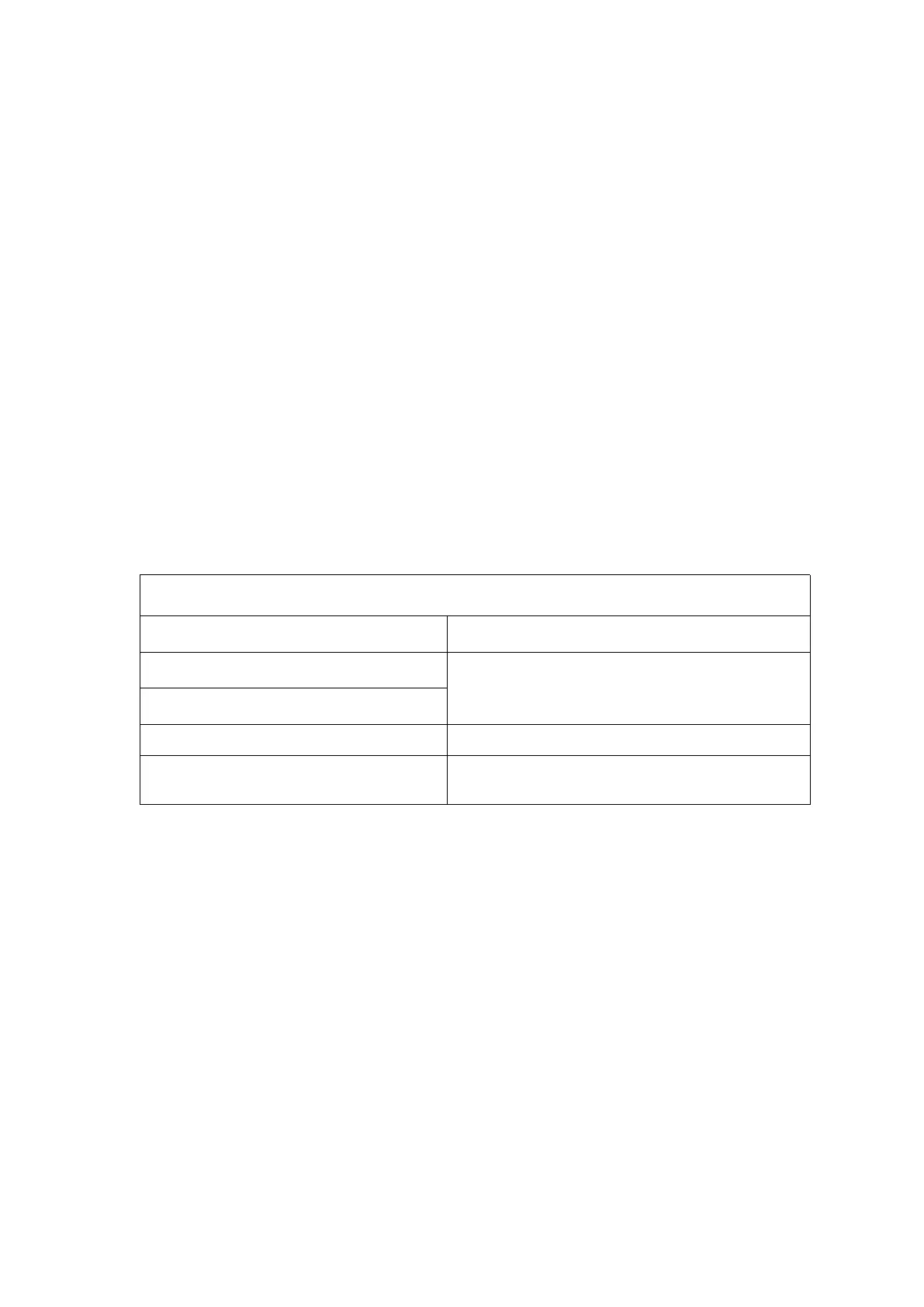
Table I: Electromagnetic emission
CISPR 11 conducted emission
CISPR 11 radiated emission
IEC61000-3-2 harmonic emission
IEC61000-3-3 voltage fluctuation/scintillation
emission
 Loading...
Loading...