Cadence II Fetal Monitor Service Manual
- 46 -
The fetal spiral electrode should not be applied to the fetal face, fontanels or genitalia.
Do not apply when placenta previa is present; when the mother has visible genital
herpes lesions or reports symptoms of prodromal lesions; when the mother is HIV
sero-positive; when mother is a confirmed carrier of hemophilia and the fetus is affected
or of unknown status; or when it is not possible to identify fetal presenting part where
application is being considered. Application when fetus is extremely premature, or in the
presence of a maternal infection such as Hepatitis B, Group B hemolytic strep, syphilis
or gonorrhea is not recommended but may be acceptable if a clear benefit to the fetus or
mother can be established.
Parts Required
① Fetal ECG cable ② Disposable fetal spiral electrode ③ Attachment pad
Operation Procedure
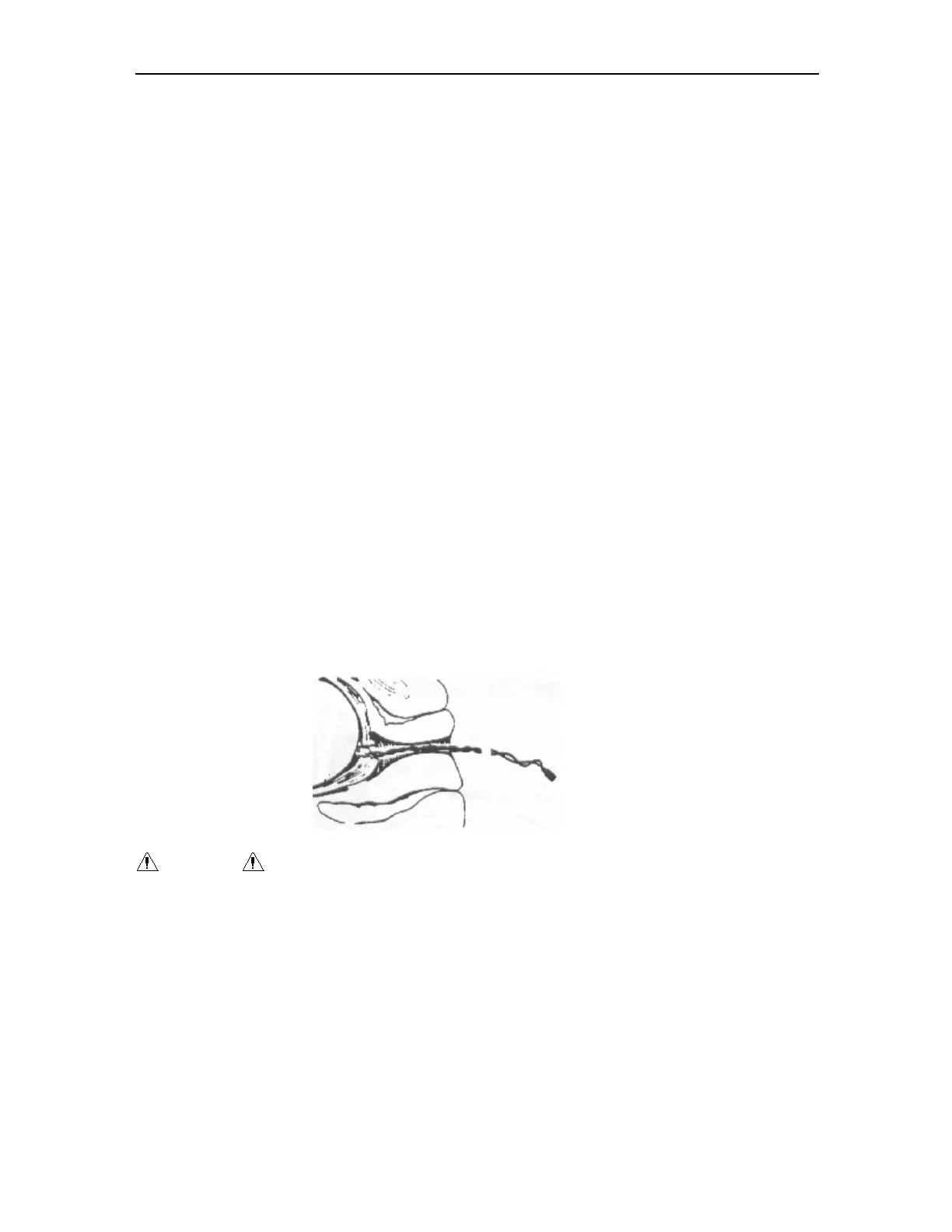
① Perform a vaginal examination and clearly identify the fetal presenting part. Using a
sterile technique to attach the fetal spiral electrode to the fetal presenting part as
described in the Directions for Use of Fetal Spiral Electrode at this section.
Figure 5-2 Connection for fetal spiral electrode
WARNING : Do not plug the fetal spiral electrode wire into the power socket.
② Fix an attachment pad at fetal ECG cable.
③ Thoroughly clean is on patient’s thigh and ensure that it is dry. Remove the release
liner from the back of the pad. Place the pad on maternal thigh and press firmly in
place (Read Prepare the Patient's Skin Prior to Placing Electrodes at this section
first).
④ Connect fetal spiral electrode with fetal ECG cable.
⑤ Switch on the power of the monitor.
 Loading...
Loading...