Vscan Extend – User Manual i-1
5721203-100 Rev. D
Revision History
Reason for Change
Please verify that you are using the latest revision of this document. Information
pertaining to this document is maintained on ePDM (GE electronic Product Data
Management). If you need to know the latest revision, contact your distributor, local GE
Sales Representative or in the USA call the GE Ultrasound Clinical Answer Center at
1 800 682 5327 or 1 262 524 5698.
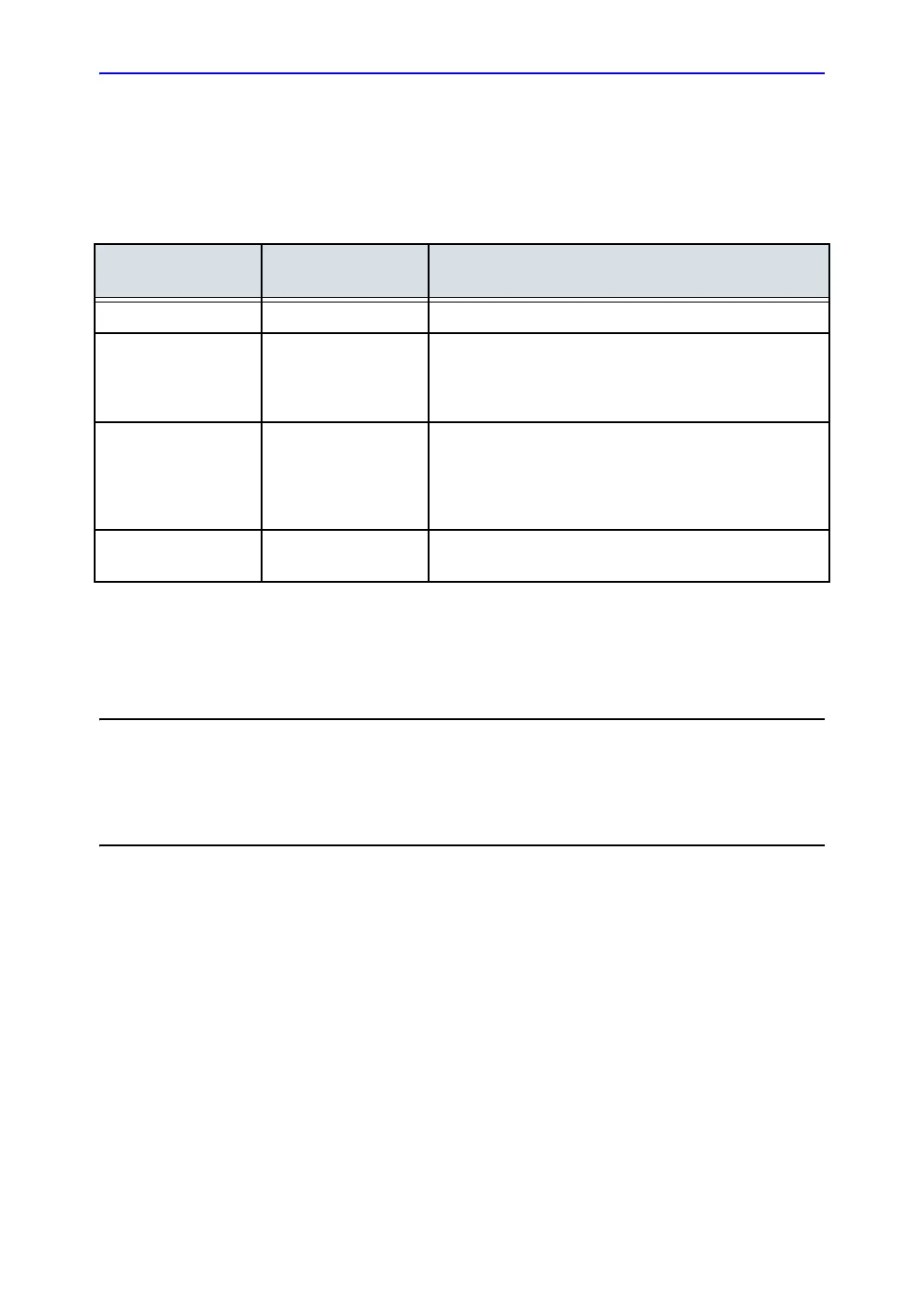
REV
DATE
(YYYY/MM/DD)
REASON FOR CHANGE
Rev. A 2016/04/05 Initial draft
Rev. B 2016/05/19 Included External Battery charger label
Included Power button under device labels
Included revised rating label
Included sections in Privacy and Security chapter
Rev. C 2016/06/01 Removed breast and testes from indication of use
statement
Included the revised rating label, battery label and
external charger label
Included the scope for all the standards in Table i-1
Rev. D 2016/08/18 Included IP33, Ophthalmic and Aorta presets, IEC
60601-11 and 12 standards, Tricefy App
 Loading...
Loading...