1-1
Chapter 1 INTRODUCTION
Please read all information carefully.
WARNING: FAILURE TO FOLLOW THE INSTRUCTIONS MAY LEAD TO SERIOUS SURGICAL
CONSEQUENCES.
All information in this guide applies to Stereotactic Guided Procedures (ST) and Ultrasound Guided
Procedures (U/S, EX) unless otherwise stated.
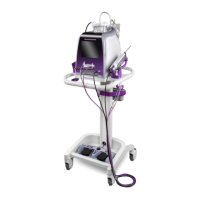
IMPORTANT: This document is designed to provide instructions for use of the Mammotome revolve Dual
Vacuum-Assisted Biopsy System. It is not a reference to surgical technique. Access to the
internet and a .pdf viewer is required to view the full electronic Instructions for Use.
NOTE: The information in this guide is subject to change without notice.
Indications
The Mammotome revolve Dual Vacuum Assisted Biopsy (VAB) System is indicated to provide tissue samples for
diagnostic sampling of breast abnormalities.
• The Mammotome revolve Dual Vacuum Assisted Biopsy (VAB) System is intended to provide breast tissue
for histologic examination with partial or complete removal of the imaged abnormality.
• The Mammotome revolve Dual Vacuum Assisted Biopsy (VAB) System is intended to provide breast tissue
for histologic examination with partial removal of a palpable abnormality.
The extent of a histologic abnormality cannot always be readily determined from palpation or imaged appearance.
Therefore, the extent of removal of the palpated or imaged evidence of an abnormality does not predict the extent
of removal of a histologic abnormality, e.g., malignancy. When the sampled abnormality is not histologically
benign, it is essential that the tissue margins be examined for completeness of removal using standard surgical
procedures.
In instances when a patient presents with a palpable abnormality that has been classified as benign through
clinical and/or radiological criteria (e.g., fibroadenoma, fibrocystic lesion), the Mammotome revolve Dual Vacuum
Assisted Biopsy (VAB) System may also be used to partially remove such palpable lesions. Whenever breast
tissue is removed, histological evaluation of the tissue is the standard of care. When the sampled abnormality is
not histologically benign, it is essential that the tissue margins be examined for completeness of removal using
standard surgical procedures.
Contraindications
• This device is not intended for use except as indicated.
• The instrument is contraindicated for those patients where increased risk or complications may be
associated with the percutaneous removal of tissue samples based upon the physician’s judgment.
Patients receiving anticoagulant therapy or who may have bleeding disorders may be at increased risk.
Warnings
Please read all the contents of the User Instructions and Operations Guide for your Mammotome revolve Dual
Vacuum-Assisted Biopsy System prior to installation and operation. Follow all warnings and instructions as stated
in this guide and retain this guide for future reference.
 Loading...
Loading...